Label: PHEXXI- lactic acid, l-, citric acid monohydrate, and potassium bitartrate gel
- NDC Code(s): 69751-100-01, 69751-100-03, 69751-100-12
- Packager: Evofem, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PHEXXI ® safely and effectively. See full prescribing information for PHEXXI. PHEXXI (lactic acid, citric acid, and potassium ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPHEXXI is indicated for the prevention of pregnancy in females of reproductive potential for use as an on-demand method of contraception. Limitations of Use - PHEXXI is not effective for the ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Administer one pre-filled applicator of PHEXXI (5 grams) vaginally immediately before or up to one hour before each act of vaginal intercourse. If more than one act of ...
-
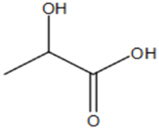
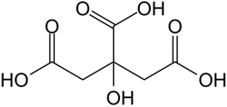
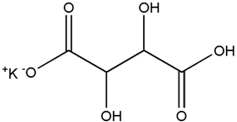
3 DOSAGE FORMS AND STRENGTHSVaginal gel: 18 mg of lactic acid, 10 mg of citric acid, and 4 mg of potassium bitartrate in each gram (1.8%, 1%, and 0.4%, respectively) of off-white to tan color gel supplied in a pre-filled ...
-
5 WARNINGS AND PRECAUTIONS5.1 Cystitis and Pyelonephritis - Among 2804 subjects who received PHEXXI in Studies 1 and 2, 0.36% (n=10) reported adverse reactions of cystitis, pyelonephritis, or other upper urinary tract ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Cystitis and Pyelonephritis [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There is no use for PHEXXI in pregnancy; therefore, discontinue PHEXXI during pregnancy. There are no data with the use of PHEXXI in pregnant women or animals ...
-
11 DESCRIPTIONPHEXXI (lactic acid, citric acid, and potassium bitartrate) is a vaginal gel. PHEXXI is an off-white to tan in color gel of uniform consistency, containing three active ingredients: lactic acid ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - In in vitro studies, Phexxi produced a normal vaginal pH range (pH 3.5 – 4.5) in the presence of semen. In clinical studies, post-coital testing demonstrated pH < 5 in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term carcinogenicity studies have not been performed with PHEXXI. Mutagenesis - Mutagenic studies have not ...
-
14 CLINICAL STUDIESThe efficacy of PHEXXI for the prevention of pregnancy was evaluated in a multi-center, open-label, single-arm clinical trial in the United States (AMP002; NCT03243305). The study enrolled females ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPHEXXI (lactic acid, citric acid, and potassium bitartrate) vaginal gel is an off-white to tan color gel of uniform consistency containing lactic acid (1.8%), citric acid (1%), and potassium ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the Patient Information and FDA-approved patient labeling (Instructions for Use). Advise the patient: To intravaginally administer the contents of one pre-filled ...
-
SPL UNCLASSIFIED SECTIONManufactured for Evofem, Inc., a wholly owned subsidiary of Evofem Biosciences, Inc., 12636 High Bluff Drive, Suite 400, San Diego, CA 92130 - ©2023 Evofem, Inc. All rights reserved. U.S. Patents ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION PHEXXI® (FEX ee) (lactic acid, citric acid, and potassium bitartrate) vaginal gel - For Vaginal Use Only - This Patient Information has been approved by the U.S. Food ...
-
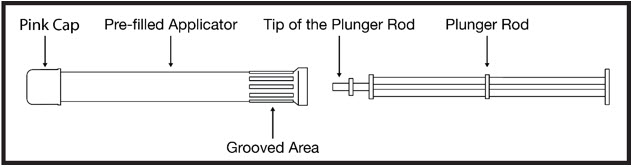
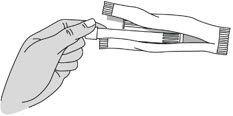
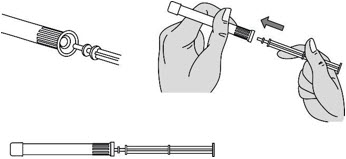
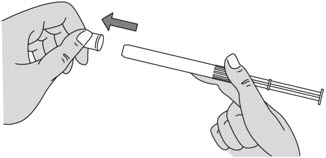
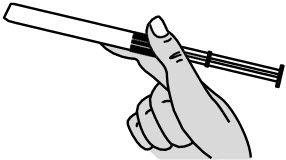
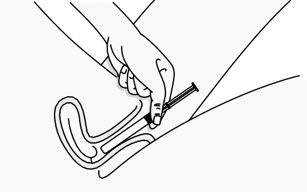
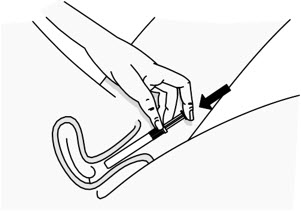
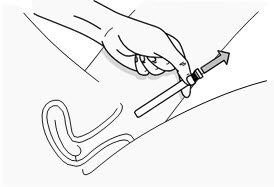
INSTRUCTIONS FOR USEPHEXXI® (FEX ee) (lactic acid, citric acid, and potassium bitartrate) vaginal gel - For Vaginal Use Only - These Instructions for Use contain information on how to use PHEXXI vaginal gel. Make ...
-
PRINCIPAL DISPLAY PANEL - 12 Applicator Boxphexxi™ (lactic acid, citric acid, and - potassium bitartrate) Vaginal Gel - 1.8%, 1%, 0.4% NDC 69751-100-12 - PN-5011
-
INGREDIENTS AND APPEARANCEProduct Information