Label: TURALIO- pexidartinib hydrochloride capsule
- NDC Code(s): 65597-407-20, 65597-407-28
- Packager: Daiichi Sankyo Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TURALIO safely and effectively. See full prescribing information for TURALIO. TURALIO® (pexidartinib) capsules, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HEPATOTOXICITY
- TURALIO can cause serious and potentially fatal liver injury, including vanishing bile duct syndrome [see Warnings and Precautions (5.1)].
- Monitor liver tests prior to initiation of TURALIO and at specified intervals during treatment. Withhold and dose reduce or permanently discontinue TURALIO based on severity of hepatotoxicity. Monitoring and prompt cessation of TURALIO may not eliminate the risk of serious and potentially fatal liver injury [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
- TURALIO is available only through a restricted program called the TURALIO Risk Evaluation and Mitigation Strategy (REMS) Program [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGETURALIO is indicated for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and not amenable to ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of TURALIO is 250 mg taken orally twice daily with a low-fat meal (approximately 11 to 14 grams of total fat) until disease progression or ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 125 mg, size 1 with white opaque body and powder blue opaque cap with black print "DSC521"
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity - TURALIO can cause serious and potentially fatal liver injury and is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) [see ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hepatotoxicity [see Warnings and Precautions (5.1)]. 6.1 Clinical Trials Experience - Because ...
-
7 DRUG INTERACTIONS7.1 Use with Hepatotoxic Products - TURALIO can cause hepatotoxicity. In patients with increased serum transaminases, total bilirubin, or direct bilirubin (>ULN) or active liver or biliary tract ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], TURALIO may cause embryo-fetal harm when administered to a ...
-
10 OVERDOSAGEDue to the high plasma protein binding, TURALIO is not expected to be dialyzable [see Clinical Pharmacology (12.3)].
-
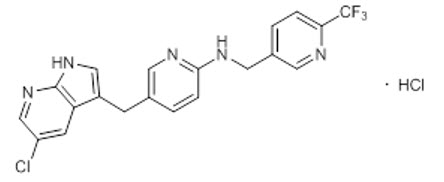
11 DESCRIPTIONPexidartinib is a kinase inhibitor. The chemical name of pexidartinib hydrochloride is 5-[(5-Chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-N-{[6-(trifluoromethyl)pyridin-3-yl]methyl}pyridin-2-amine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pexidartinib is a small molecule tyrosine kinase inhibitor that targets colony stimulating factor 1 receptor (CSF1R), KIT proto-oncogene receptor tyrosine kinase (KIT) ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies were performed in mice and rats. Both studies were negative for carcinogenic findings at exposures up to 9 ...
-
14 CLINICAL STUDIES14.1 Tenosynovial Giant Cell Tumor - The efficacy of TURALIO 250 mg orally twice daily administered with a low-fat meal has been established based on adequate and well-controlled studies of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTURALIO 125 mg capsules are supplied as size 1 with white opaque body and powder blue opaque cap with black print "DSC521", available in: 28 count bottle - 120 count bottle - NDC# ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Hepatotoxicity - Advise patients of the risk of hepatotoxicity that could be fatal and that they will need to ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Daiichi Sankyo, Inc. Basking Ridge, NJ 07920 - TURALIO® is a registered trademark of Daiichi Sankyo Company, Limited. ©2025, Daiichi Sankyo, Inc. USPI-TUR125-C8-0125-r102
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: 1/2025 Medication Guide - TURALIO® (tur-a-lee-oh) (pexidartinib) capsules - What is the most ...
-
PRINCIPAL DISPLAY PANEL - 125 mg Capsule Bottle CartonNDC 65597-407-20 - Rx only - Turalio® (pexidartinib) capsules - 125 mg - 120 capsules - Take with a low-fat meal. Pharmacist: Dispense the - Medication Guide to each patient. Daiichi-Sankyo
-
PRINCIPAL DISPLAY PANEL - 125 mg Capsule Bottle Carton - 65597-407-28NDC 65597-407-28 - Rx only - Turalio® (pexidartinib) capsules - 125 mg - 28 capsules - Take with a low-fat meal. Pharmacist: Dispense the - Medication Guide to each patient. Daiichi-Sankyo
-
INGREDIENTS AND APPEARANCEProduct Information