Label: SOMAVERT- pegvisomant kit
- NDC Code(s): 0009-5175-02, 0009-5177-02, 0009-5179-02, 0009-5201-04, view more
- Packager: Pharmacia & Upjohn Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOMAVERT safely and effectively. See full prescribing information for SOMAVERT. SOMAVERT (pegvisomant) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESOMAVERT is indicated for the treatment of acromegaly in patients who have had an inadequate response to surgery or radiation therapy, or for whom these therapies are not appropriate. The goal of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage Information - The recommended loading dose of SOMAVERT is 40 mg given subcutaneously, under healthcare provider supervision. Provide proper training in subcutaneous injection technique ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 10 mg, 15 mg, 20 mg, 25 mg or 30 mg white lyophilized powder in a single-dose vial for reconstitution supplied with a prefilled syringe containing 1 mL of diluent (Sterile Water for ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypoglycemia Associated With GH Lowering in Patients With Diabetes Mellitus - GH opposes the effects of insulin on carbohydrate metabolism by decreasing insulin sensitivity; thus, glucose ...
-
6 ADVERSE REACTIONSClinically significant adverse reactions that appear in other section of the labeling include: • Hypoglycemia Associated with GH Lowering in Patients with Diabetes Mellitus [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Insulin and/or Oral Hypoglycemic Agents - After initiation of SOMAVERT, patients with acromegaly and diabetes mellitus treated with insulin and/or oral hypoglycemic agents may require dose ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Postmarketing reports of SOMAVERT use in pregnant women are insufficient to establish a drug-associated risk for major birth defects, miscarriage or adverse ...
-
10 OVERDOSAGEIn one reported incident of acute overdose with SOMAVERT during pre-marketing clinical studies, a patient self-administered 80 mg/day (2.7 times the maximum recommended maintenance dosage) for ...
-
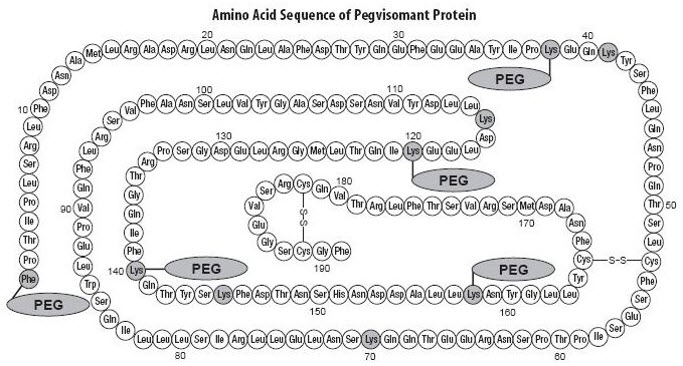
11 DESCRIPTIONPegvisomant is an analog of human growth hormone (GH) of recombinant DNA origin that acts as a GH receptor antagonist. It contains 191 amino acid residues. The molecular weight of pegvisomant is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pegvisomant selectively binds to growth hormone (GH) receptors on cell surfaces, where it blocks the binding of endogenous GH, and thus interferes with GH signal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Pegvisomant was administered subcutaneously to rats daily for 2 years at doses of 2, 8, and 20 mg/kg (about 2, 9 ...
-
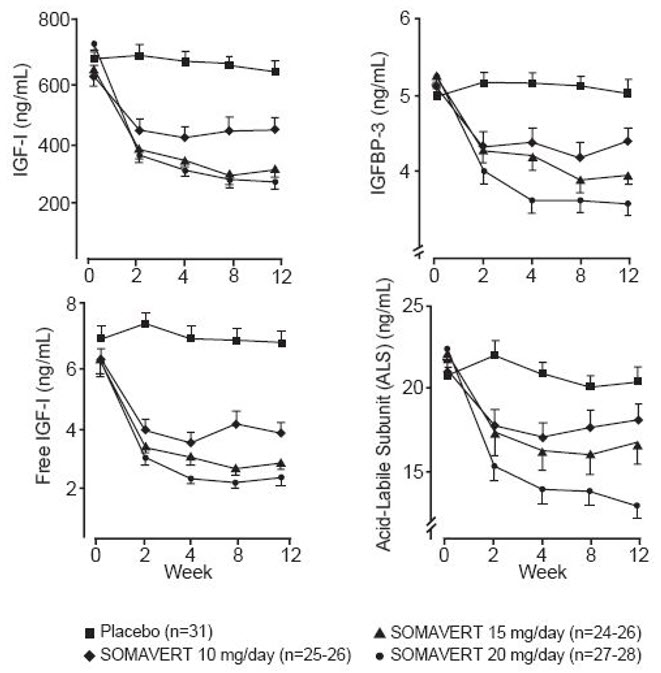
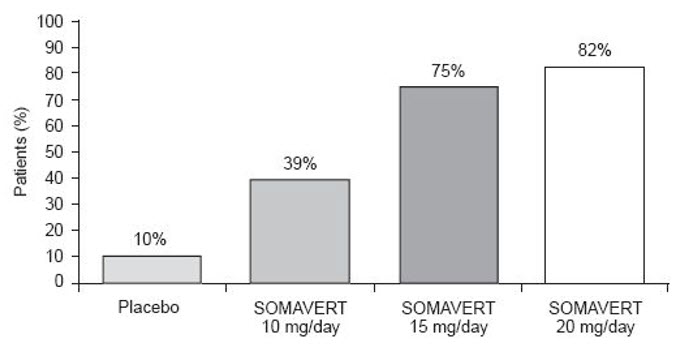
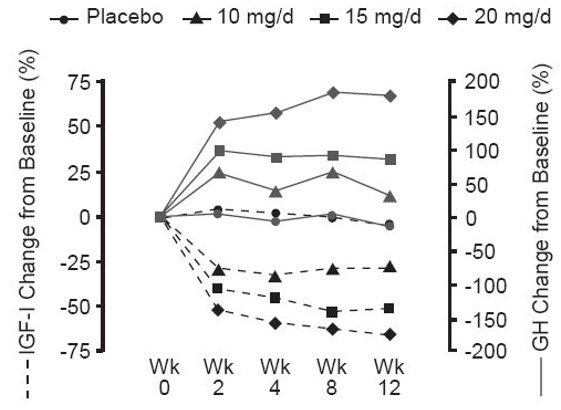
14 CLINICAL STUDIESA total of one hundred twelve patients (63 men and 49 women) with acromegaly participated in a 12-week, randomized, double-blind, multi-center study comparing placebo and SOMAVERT. The mean ±SD ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSOMAVERT (pegvisomant) for injection is a white lyophilized powder supplied in the following strengths and package configurations: One Day Package ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Inform patients (and/or their caregivers) of the following information to aid in the ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 7/2023 - PATIENT INFORMATION - SOMAVERT (SOM-ah-vert) (pegvisomant) for injection, for ...
-
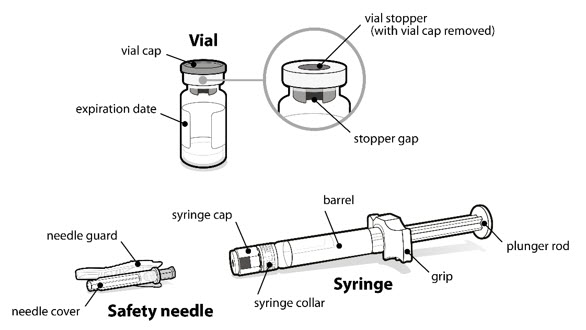
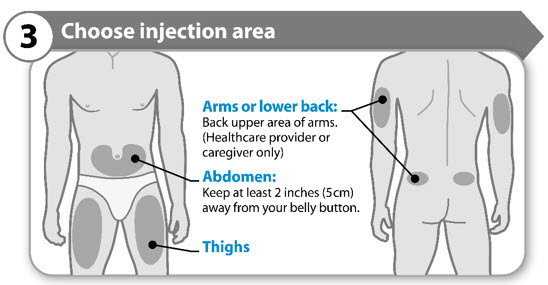
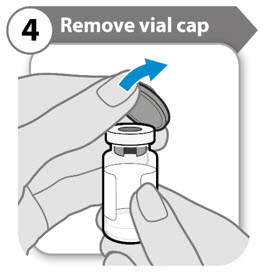
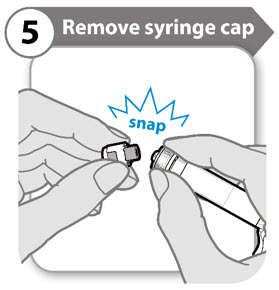
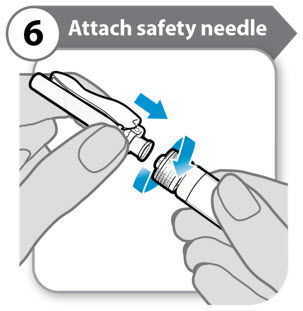
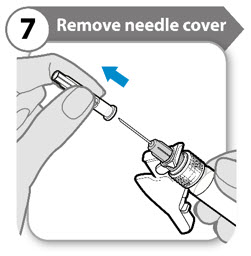
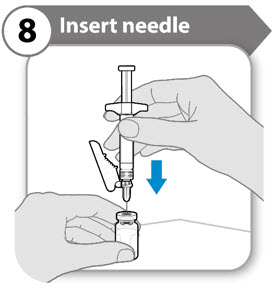
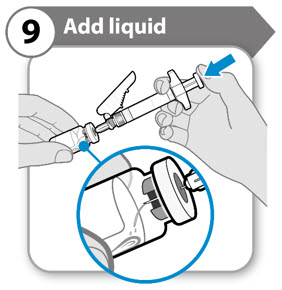
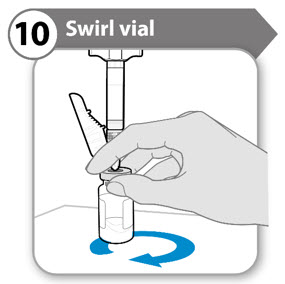
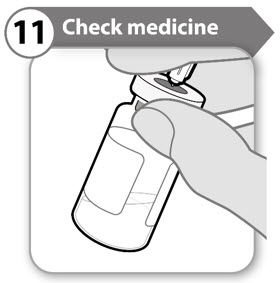
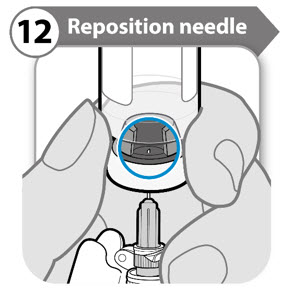
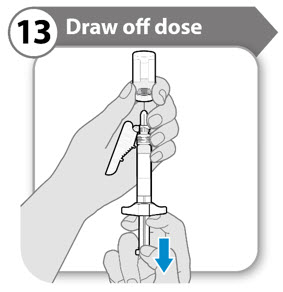
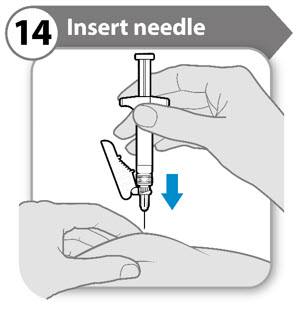
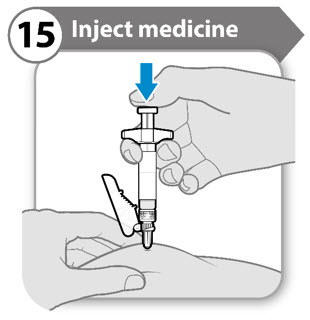
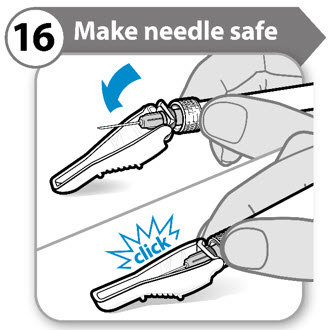
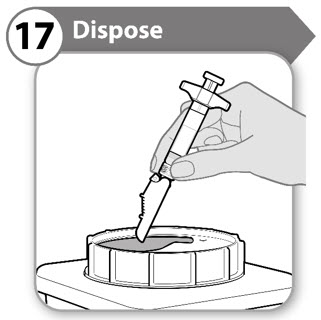
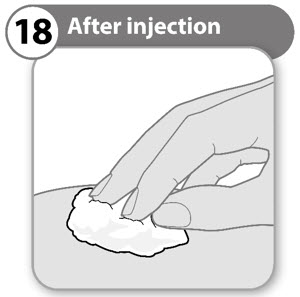
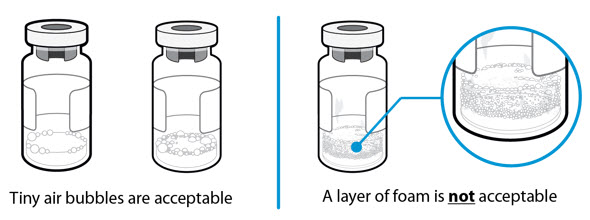
INSTRUCTIONS FOR USESOMAVERT® (SOM-ah-vert) (pegvisomant) for injection, for subcutaneous use - Read these Instructions for Use before you start using SOMAVERT and each time you get a refill. There may be new ...
-
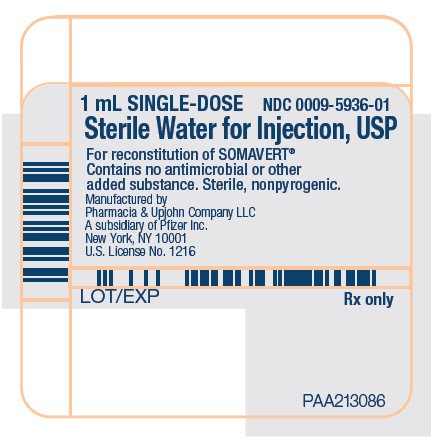
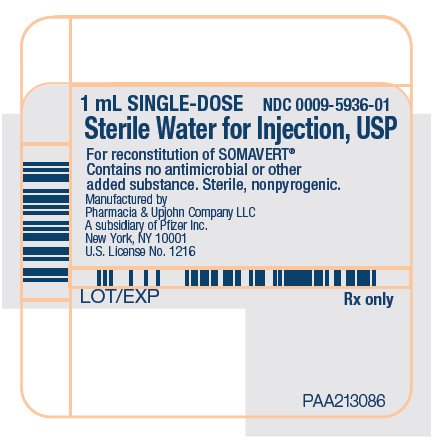
PRINCIPAL DISPLAY PANEL - 1 mL Syringe Label1 mL SINGLE-DOSE - NDC 0009-5936-01 - Sterile Water for Injection, USP - For reconstitution of SOMAVERT® Contains no antimicrobial or other - added substance. Sterile, nonpyrogenic. Manufactured ...
-
PRINCIPAL DISPLAY PANEL - 10 mg Vial LabelNDC 0009-5175-02 - Pfizer - Somavert® (pegvisomant) for injection - 10 mg/vial - For Subcutaneous Use Only - One Single-Dose Vial - Discard unused portion. Rx only - PAA213088
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 7166-01NDC 0009-7166-01 - Pfizer - Somavert® (pegvisomant) for injection - 10 mg/vial - For Subcutaneous Injection Only - One Single-Dose Vial - Discard unused portion. Contents: One Somavert single-dose vial ...
-
PRINCIPAL DISPLAY PANEL - 10 mg Vial CartonNDC 0009-5175-10 - Pfizer - Somavert® (pegvisomant) for injection - 10 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of intermediate carton: Ten ...
-
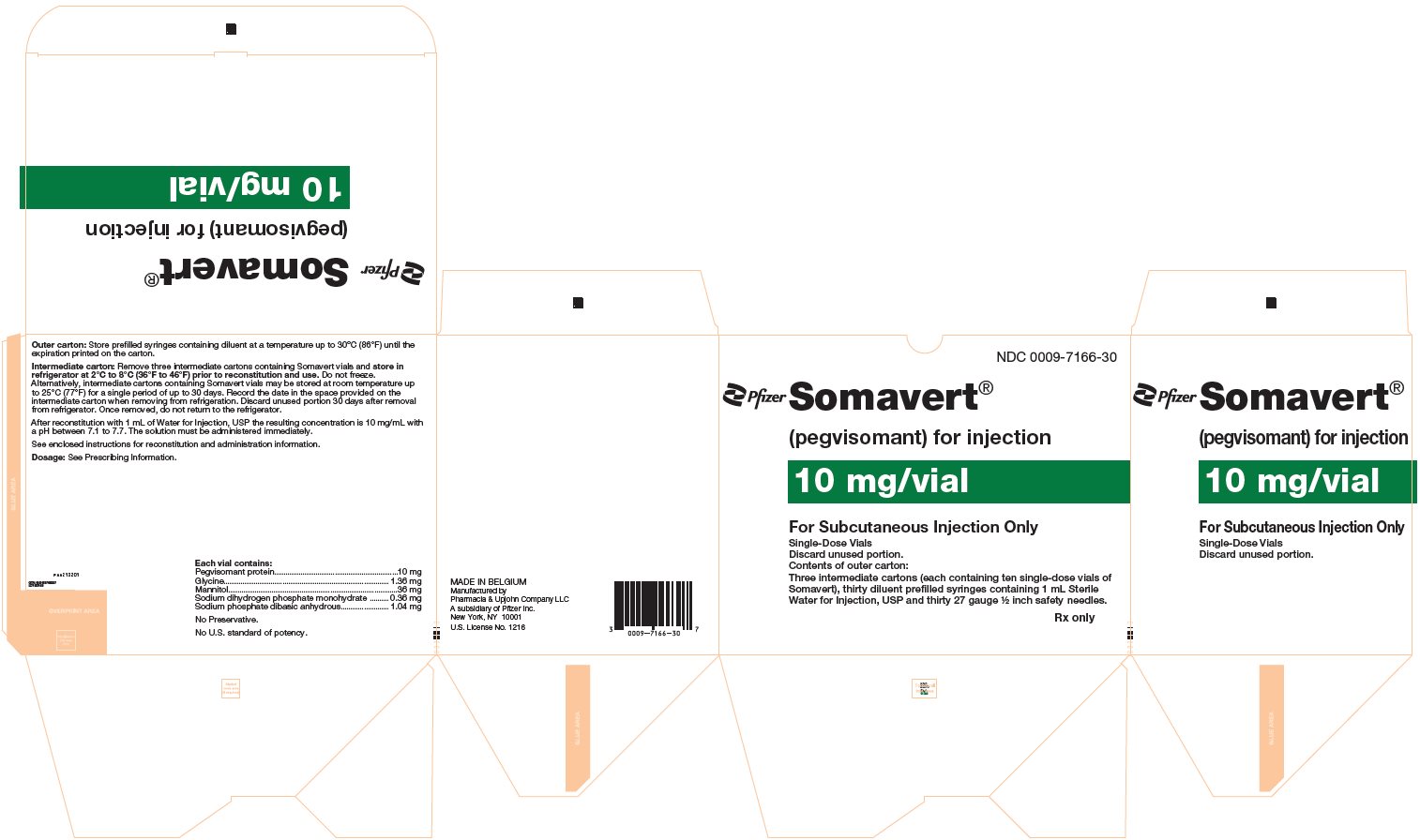
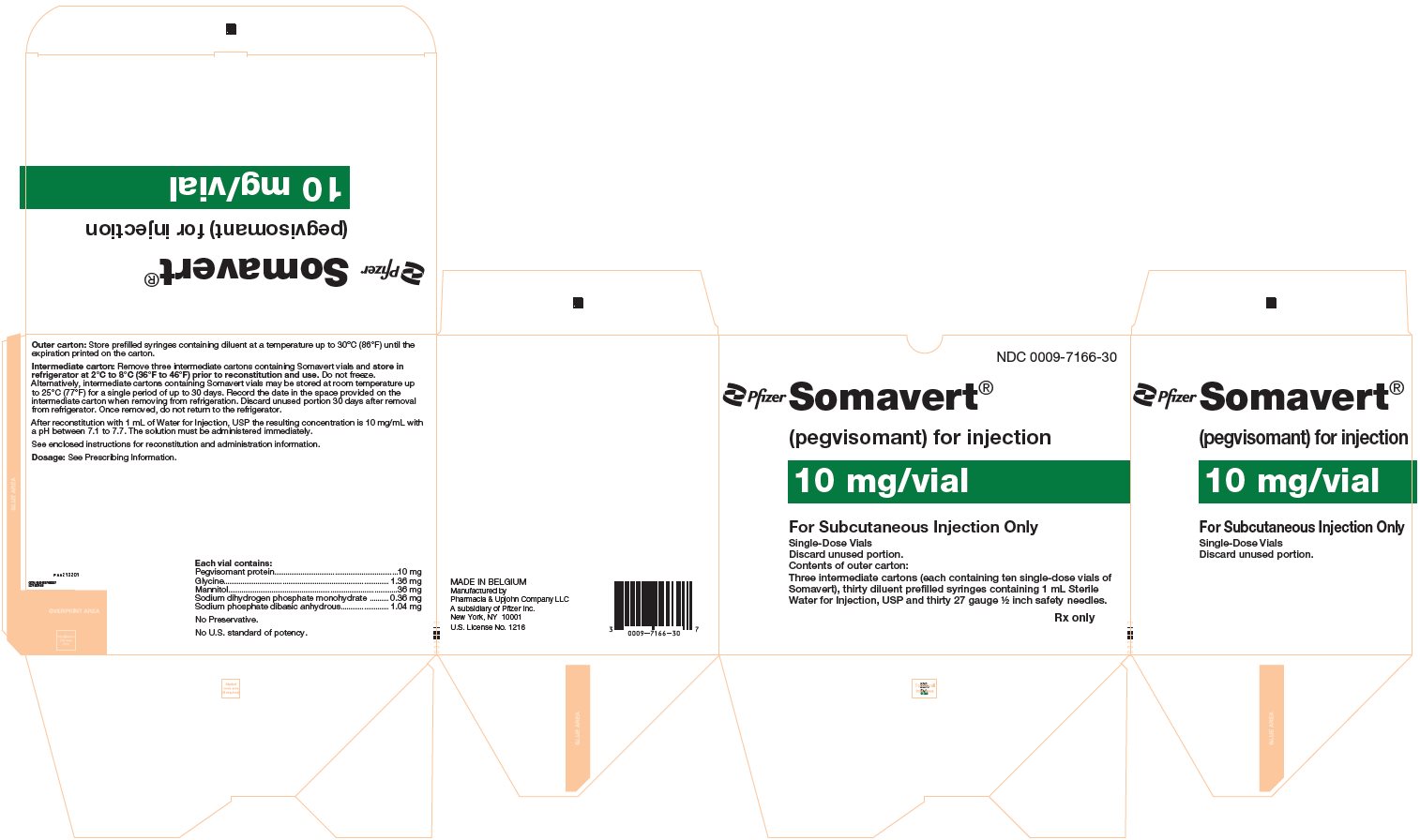
PRINCIPAL DISPLAY PANEL - Kit Carton - 7166-30NDC 0009-7166-30 - Pfizer - Somavert® (pegvisomant) for injection - 10 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of outer carton: Three intermediate ...
-
PRINCIPAL DISPLAY PANEL - 15 mg Vial LabelNDC 0009-5177-02 - Pfizer - Somavert® (pegvisomant) for injection - 15 mg/vial - For Subcutaneous Use Only - One Single-Dose Vial - Discard unused portion. Rx only - PAA213202
-
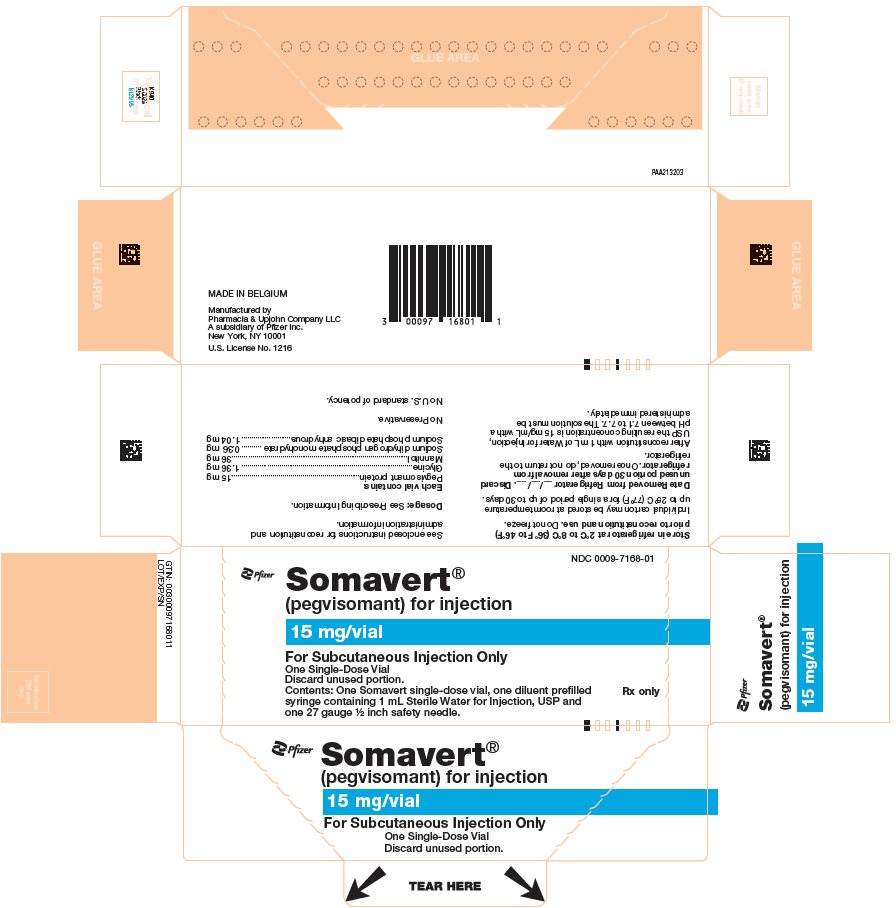
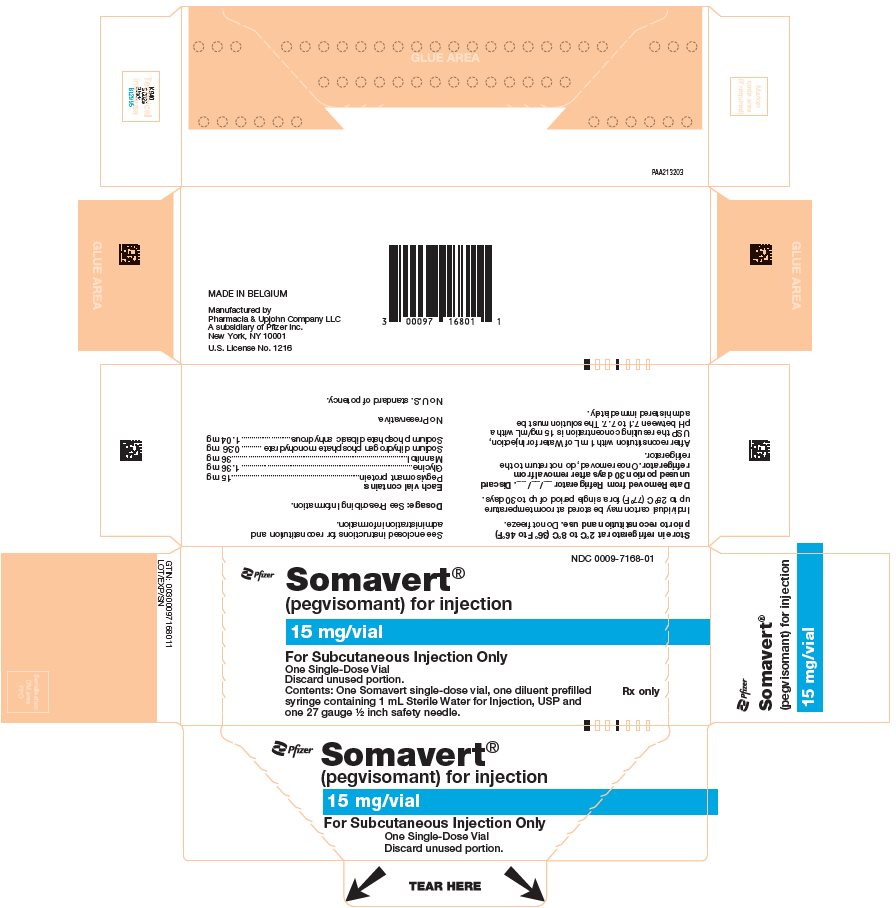
PRINCIPAL DISPLAY PANEL - Kit Carton - 7168-01NDC 0009-7168-01 - Pfizer - Somavert® (pegvisomant) for injection - 15 mg/vial - For Subcutaneous Injection Only - One Single-Dose Vial - Discard unused portion. Contents: One Somavert single-dose vial ...
-
PRINCIPAL DISPLAY PANEL - 15 mg Vial CartonNDC 0009-5177-10 - Pfizer - Somavert® (pegvisomant) for injection - 15 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of intermediate carton: Ten single-dose ...
-
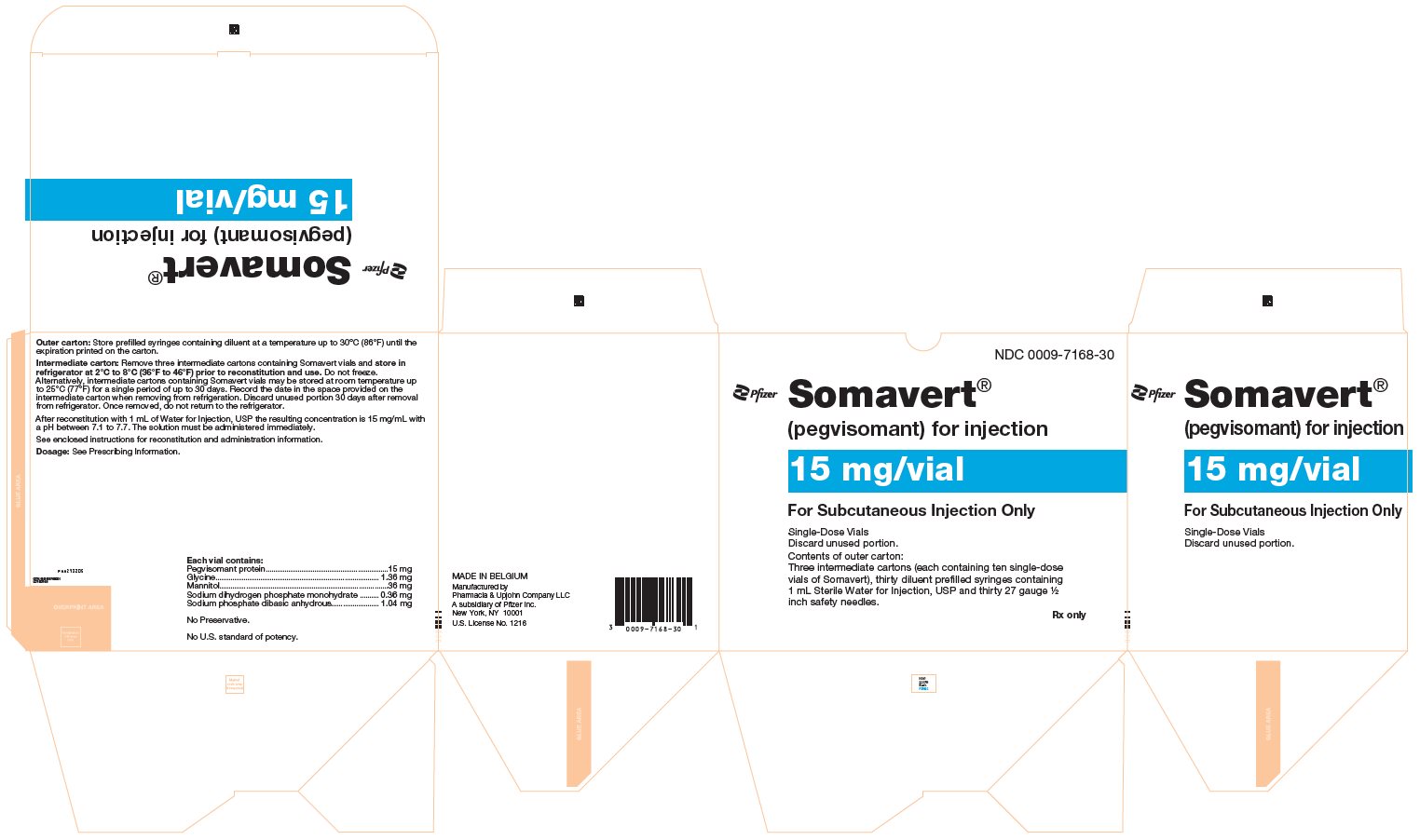
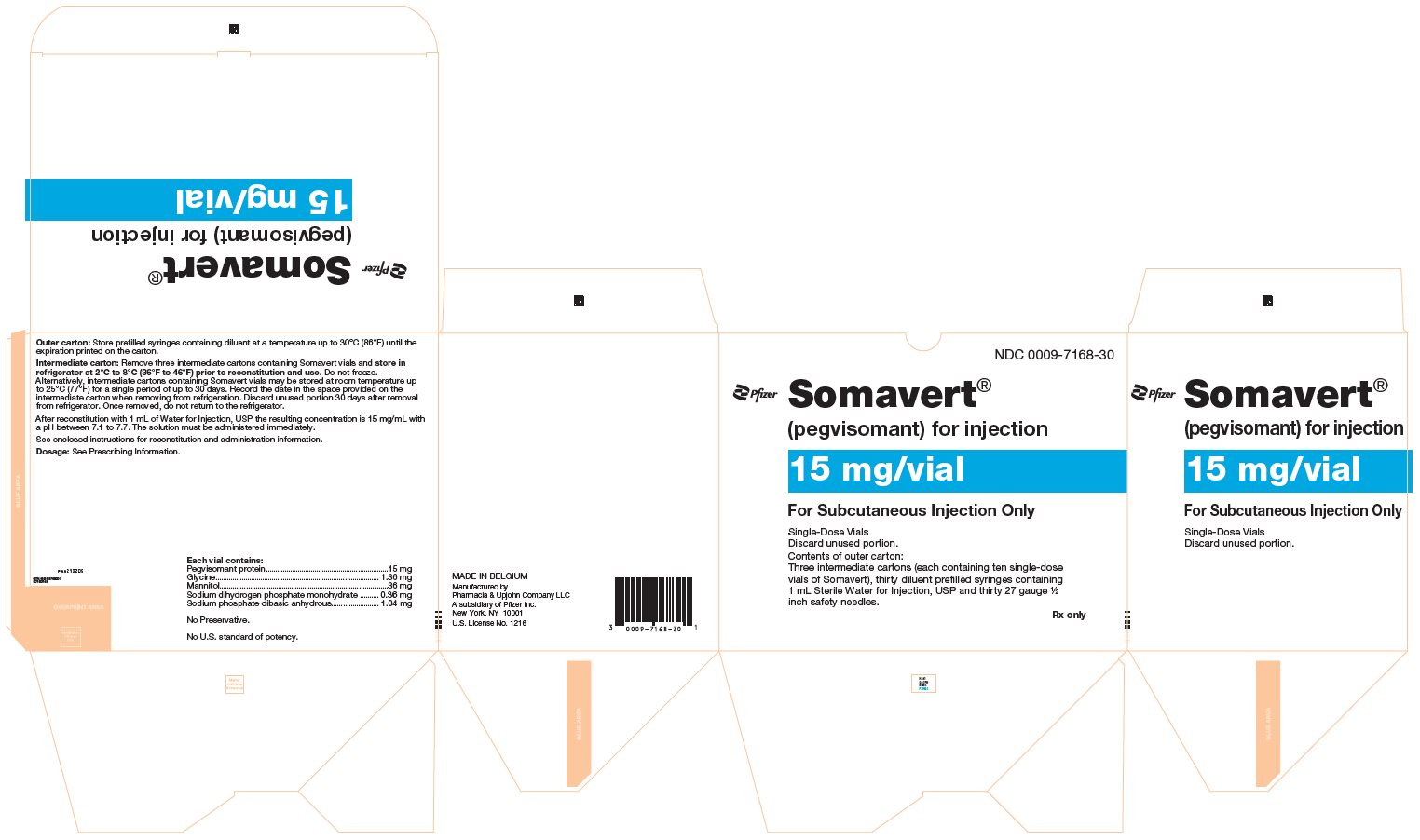
PRINCIPAL DISPLAY PANEL - Kit Carton - 7168-30NDC 0009-7168-30 - Pfizer - Somavert® (pegvisomant) for injection - 15 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of outer carton: Three intermediate ...
-
PRINCIPAL DISPLAY PANEL - 20 mg Vial LabelNDC 0009-5179-02 - Pfizer - Somavert® (pegvisomant) for injection - 20 mg/vial - For Subcutaneous Use Only - One Single-Dose Vial - Discard unused portion. Rx only - PAA213206
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 7188-01NDC 0009-7188-01 - Pfizer - Somavert® (pegvisomant) for injection - 20 mg/vial - For Subcutaneous Injection Only - One Single-Dose Vial - Discard unused portion. Contents: One Somavert single-dose vial, one ...
-
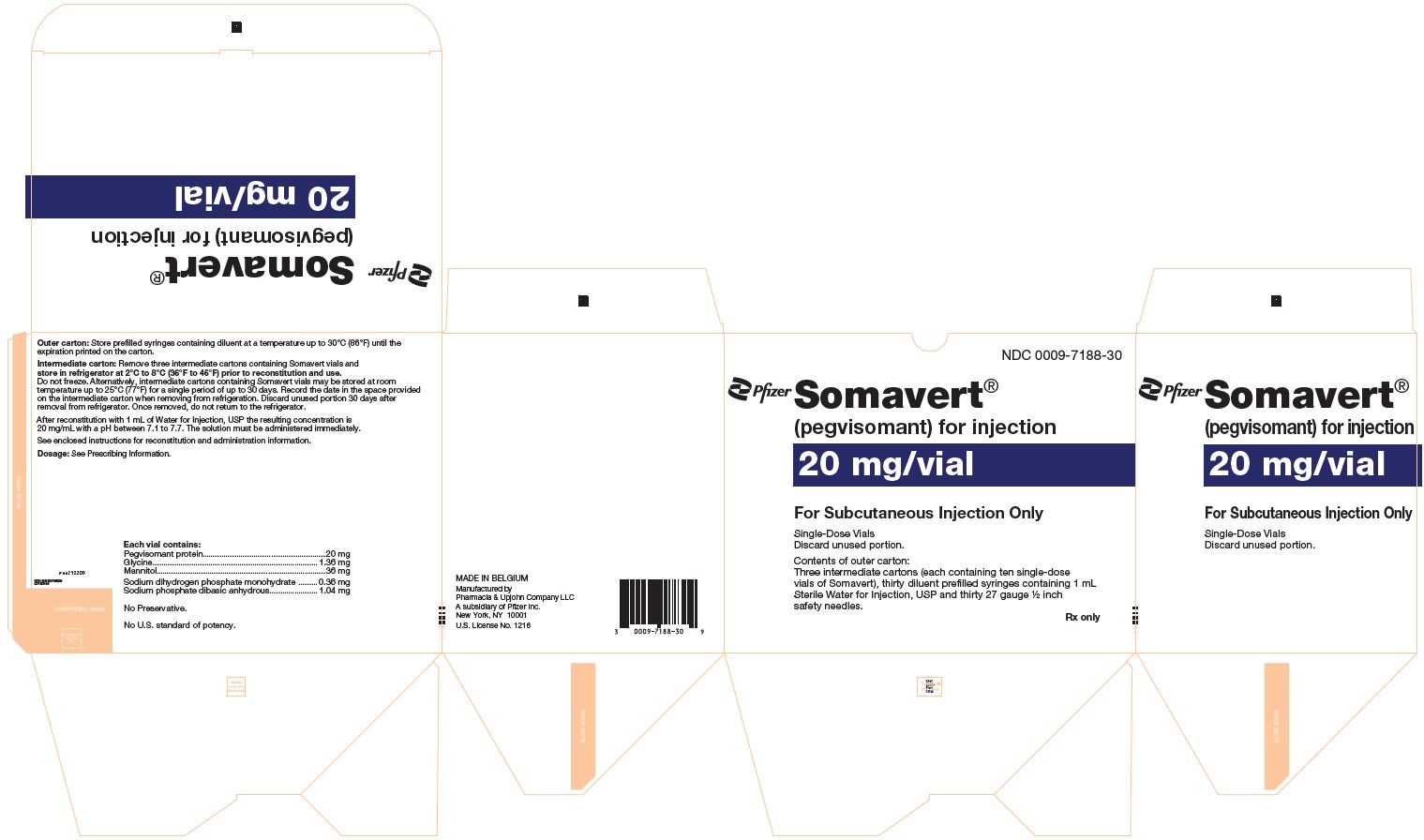
PRINCIPAL DISPLAY PANEL - 20 mg Vial CartonNDC 0009-5179-10 - Pfizer - Somavert® (pegvisomant) for injection - 20 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of intermediate carton: Ten single-dose ...
-
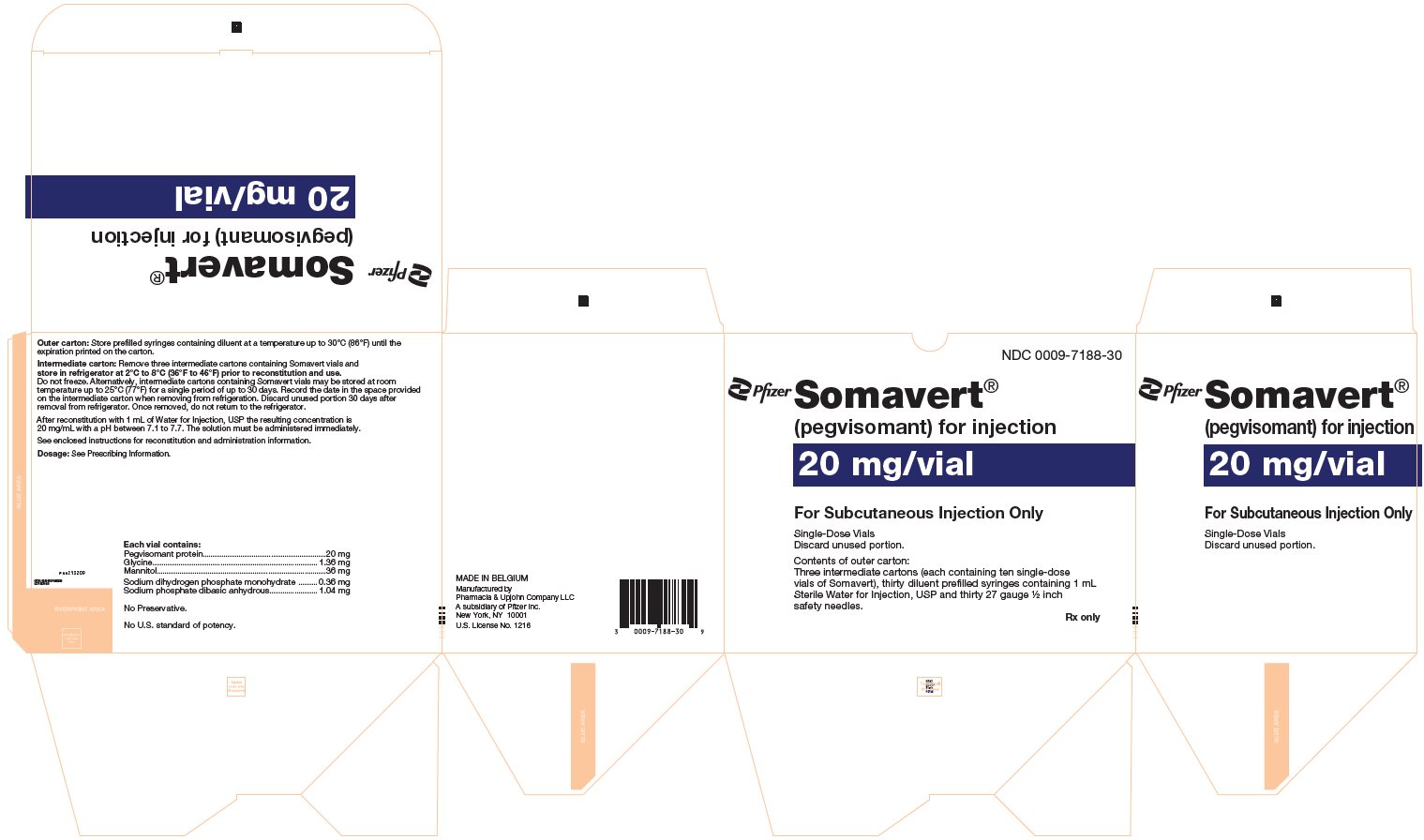
PRINCIPAL DISPLAY PANEL - Kit Carton - 7188-30NDC 0009-7188-30 - Pfizer - Somavert® (pegvisomant) for injection - 20 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of outer carton: Three intermediate ...
-
PRINCIPAL DISPLAY PANEL - 25 mg Vial LabelNDC 0009-5201-04 - Pfizer - Somavert® (pegvisomant) for injection - 25 mg/vial - For Subcutaneous Use Only - One Single-Dose Vial - Discard unused portion. Rx only - PAA213210
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 7199-01NDC 0009-7199-01 - Pfizer - Somavert® (pegvisomant) for injection - 25 mg/vial - For Subcutaneous Injection Only - One Single-Dose Vial - Discard unused portion. Contents: One Somavert single-dose vial, one ...
-
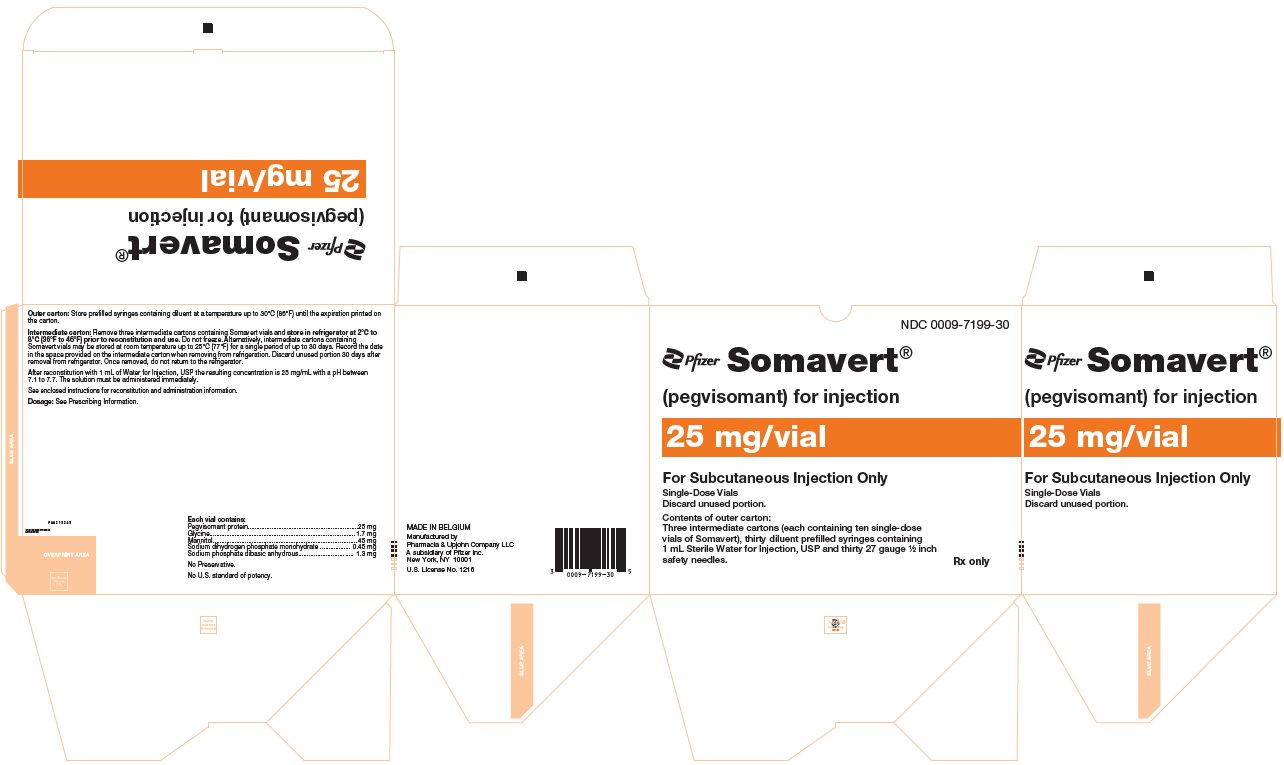
PRINCIPAL DISPLAY PANEL - 25 mg Vial CartonNDC 0009-5201-10 - Pfizer - Somavert® (pegvisomant) for injection - 25 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of intermediate carton: Ten single-dose ...
-
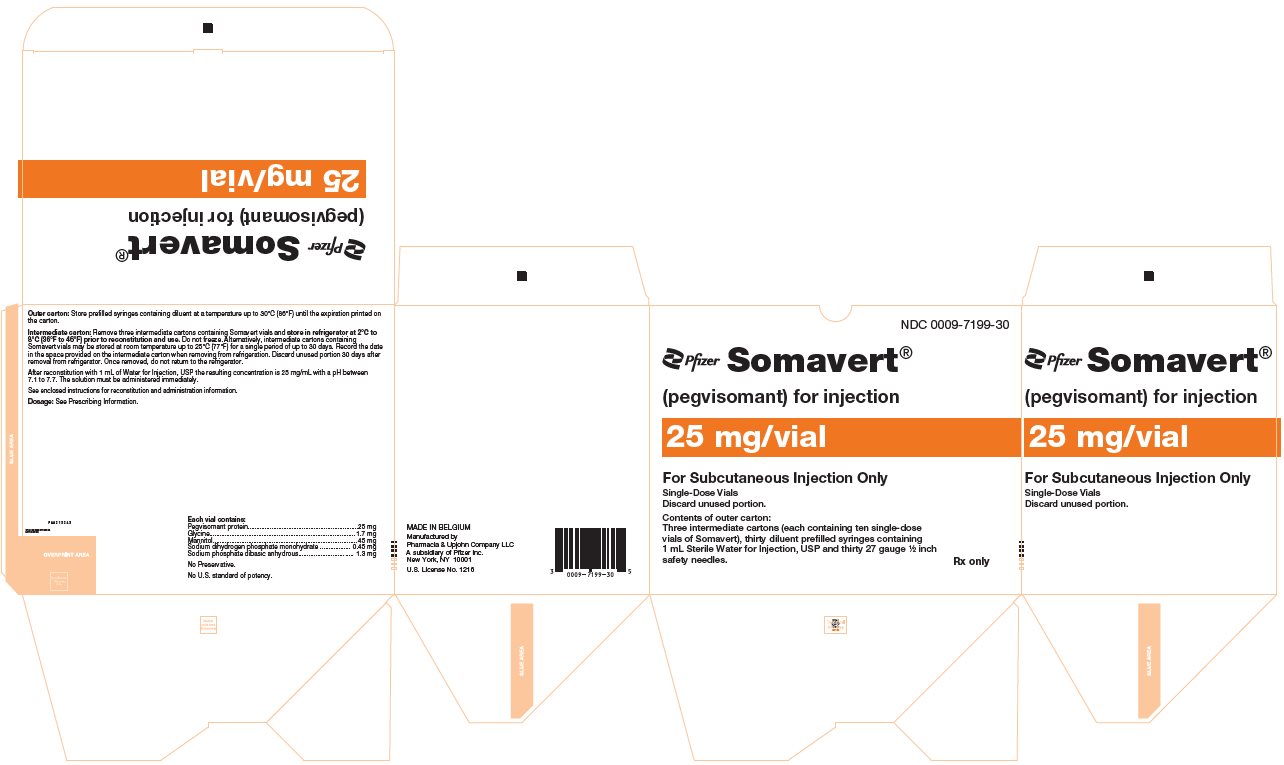
PRINCIPAL DISPLAY PANEL - Kit Carton - 7199-30NDC 0009-7199-30 - Pfizer - Somavert® (pegvisomant) for injection - 25 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of outer carton: Three intermediate ...
-
PRINCIPAL DISPLAY PANEL - 30 mg Vial LabelNDC 0009-5376-04 - Pfizer - Somavert® (pegvisomant) for injection - 30 mg/vial - For Subcutaneous Use Only - One Single-Dose Vial - Discard unused portion. Rx only - PAA213244
-
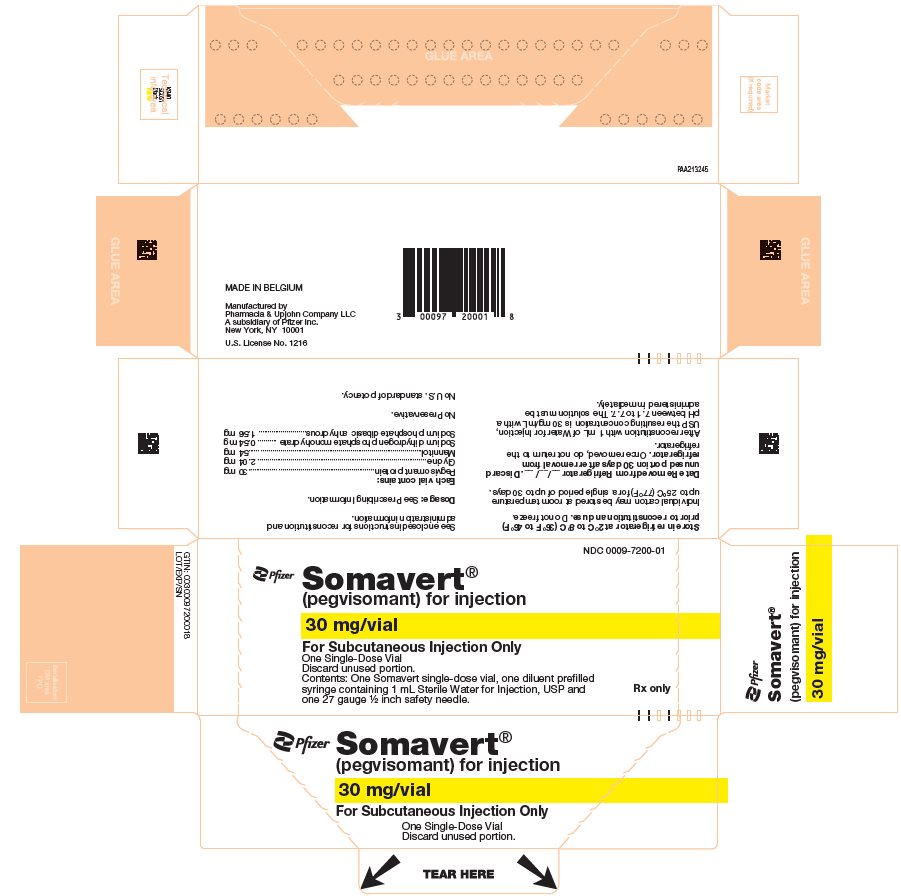
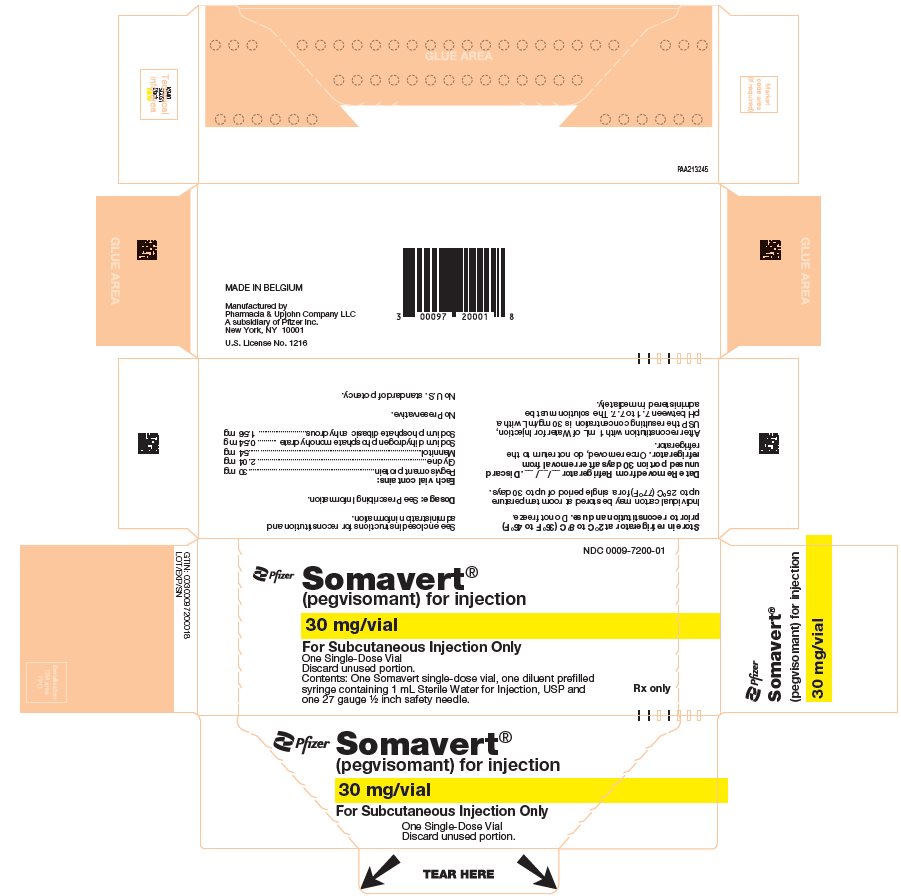
PRINCIPAL DISPLAY PANEL - Kit Carton - 7200-01NDC 0009-7200-01 - Pfizer - Somavert® (pegvisomant) for injection - 30 mg/vial - For Subcutaneous Injection Only - One Single-Dose Vial - Discard unused portion. Contents: One Somavert single-dose vial, one ...
-
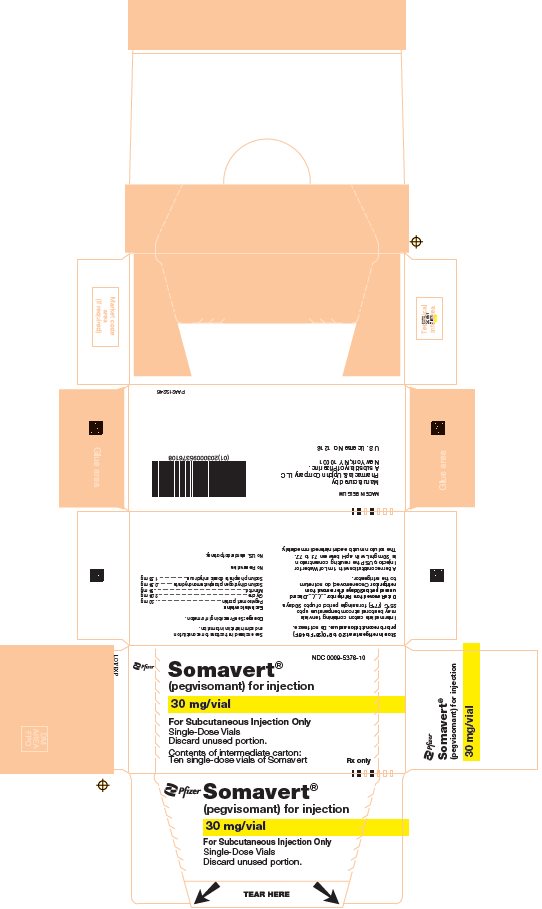
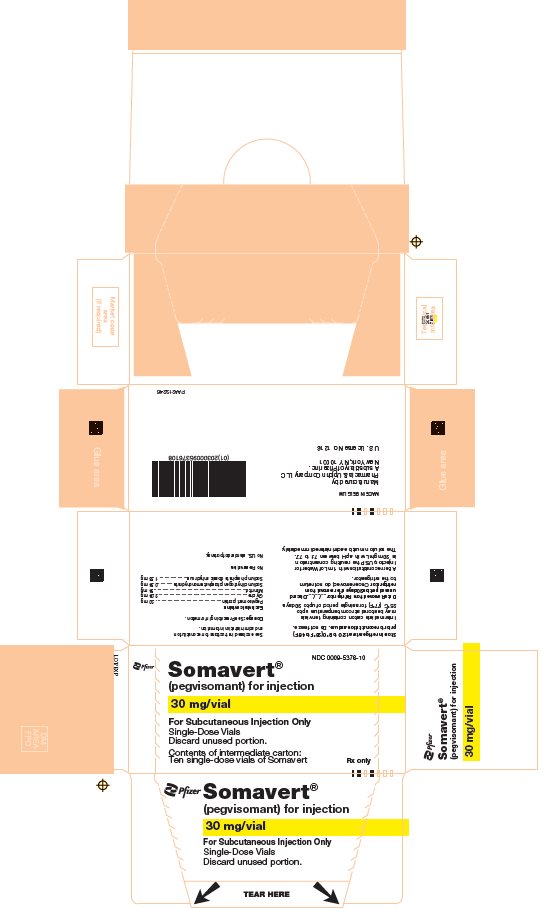
PRINCIPAL DISPLAY PANEL - 30 mg Vial CartonNDC 0009-5376-10 - Pfizer - Somavert® (pegvisomant) for injection - 30 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of intermediate carton: Ten single-dose ...
-
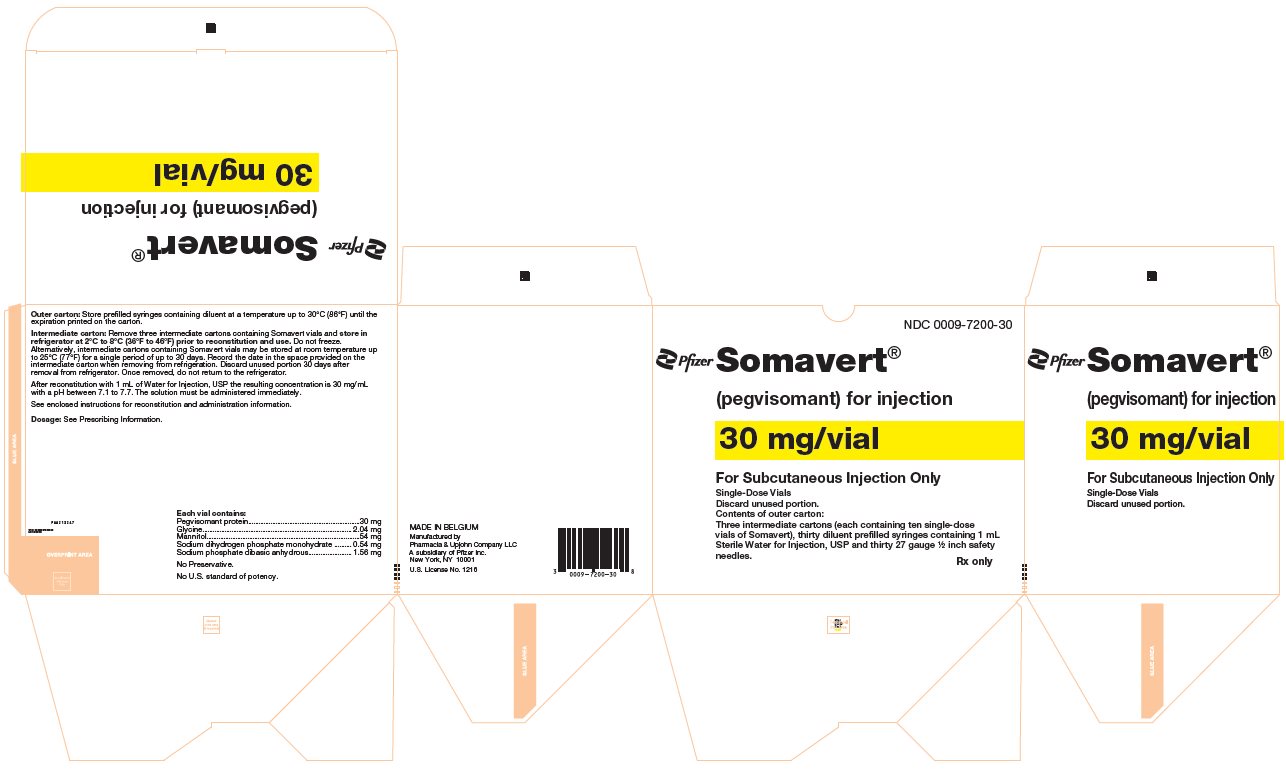
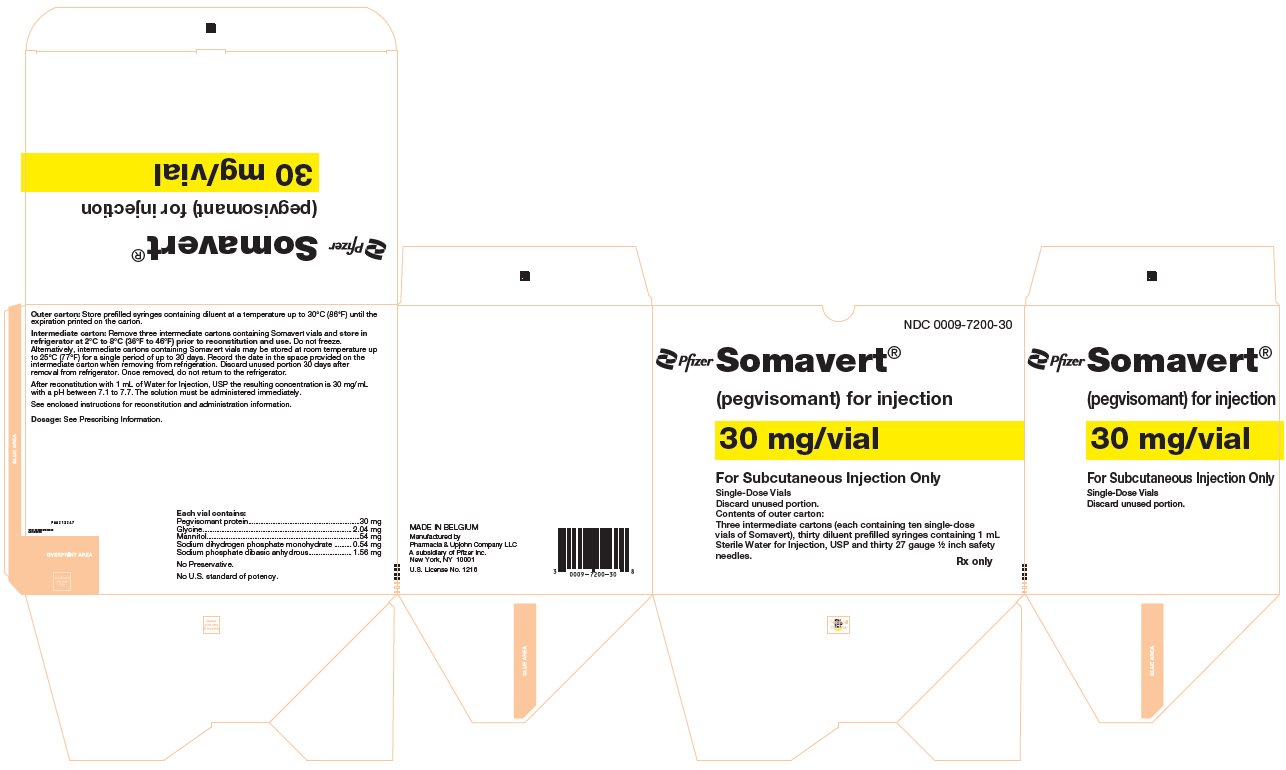
PRINCIPAL DISPLAY PANEL - Kit Carton - 7200-30NDC 0009-7200-30 - Pfizer - Somavert® (pegvisomant) for injection - 30 mg/vial - For Subcutaneous Injection Only - Single-Dose Vials - Discard unused portion. Contents of outer carton: Three intermediate ...
-
INGREDIENTS AND APPEARANCEProduct Information