Label: PALYNZIQ- pegvaliase-pqpz injection, solution
-
NDC Code(s):
68135-058-89,
68135-058-90,
68135-673-39,
68135-673-40, view more68135-673-45, 68135-756-19, 68135-756-20
- Packager: BioMarin Pharmaceutical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PALYNZIQ safely and effectively. See full prescribing information for PALYNZIQ. PALYNZIQ (pegvaliase-pqpz) injection, for ...These highlights do not include all the information needed to use PALYNZIQ safely and effectively. See full prescribing information for PALYNZIQ.
PALYNZIQ (pegvaliase-pqpz) injection, for subcutaneous use
Initial U.S. Approval: 2018WARNING: RISK OF ANAPHYLAXIS
See full prescribing information for complete boxed warning.
- Anaphylaxis has been reported after administration of Palynziq and may occur at any time during treatment. (5.1)
- Administer the initial dose of Palynziq under the supervision of a healthcare provider equipped to manage anaphylaxis, and closely observe patients for at least 60 minutes following injection. Prior to self‑injection, confirm patient competency with self‑administration, and patient’s and observer’s (if applicable) ability to recognize signs and symptoms of anaphylaxis and to administer auto-injectable epinephrine, if needed. (2.4)
- Prescribe auto‑injectable epinephrine. Prior to first dose, instruct the patient and observer (if applicable) on its appropriate use. Instruct the patient to seek immediate medical care upon its use. Instruct patients to carry auto-injectable epinephrine with them at all times during Palynziq treatment. (2.4, 5.1)
- Palynziq is available only through a restricted program called the Palynziq REMS. (5.2)
RECENT MAJOR CHANGES
Dosage and Administration (2.1) 10/2020 INDICATIONS AND USAGE
Palynziq is a phenylalanine (Phe)‑metabolizing enzyme indicated to reduce blood Phe concentrations in adult patients with phenylketonuria who have uncontrolled blood Phe concentrations greater than 600 micromol/L on existing management. (1)
DOSAGE AND ADMINISTRATION
Dosage (2.1)
- Obtain baseline blood Phe concentration before initiating treatment.
- The recommended initial dosage is 2.5 mg subcutaneously once weekly for 4 weeks.
- Titrate the dosage in a step-wise manner over at least 5 weeks based on tolerability to achieve a dosage of 20 mg once daily. See full prescribing information for titration regimen.
- Assess patient tolerability, blood Phe concentration, and dietary protein and Phe intake throughout treatment.
- Consider increasing the dosage to 40 mg once daily in patients who have been on 20 mg once daily continuously for at least 24 weeks and who have not achieved blood Phe control (blood phenylalanine concentration less than or equal to 600 micromol/L).
- Consider increasing the dosage to a maximum of 60 mg once daily in patients who have been on 40 mg once daily continuously for at least 16 weeks and who have not achieved blood Phe control.
- Discontinue Palynziq in patients who have not achieved an adequate response after 16 weeks of continuous treatment with the maximum dosage of 60 mg once daily.
- Reduce the dosage and/or modify dietary protein and Phe intake, as needed, to maintain blood Phe concentrations within a clinically acceptable range and above 30 micromol/L.
Blood Phenylalanine Monitoring and Diet (2.2)
- Obtain blood Phe concentrations every 4 weeks until a maintenance dosage is established.
- Periodically monitor blood Phe concentrations during maintenance therapy.
- Counsel patients to monitor dietary protein and Phe intake, and adjust as directed by their healthcare provider.
- Consider premedication for hypersensitivity reactions.
Administration Instructions (2.4)
- Rotate injection sites. If more than one injection is needed for a single dose, the injection sites should be at least 2 inches away from each other.
DOSAGE FORMS AND STRENGTHS
Injection: 2.5 mg/0.5 mL, 10 mg/0.5 mL, and 20 mg/mL in a single-dose prefilled syringe. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions, Other than Anaphylaxis: Management should be based on the severity of the reaction, recurrence, and clinical judgement, and may include dosage adjustment, temporary drug interruption, or treatment with antihistamines, antipyretics, and/or corticosteroids. (5.3)
ADVERSE REACTIONS
Most common adverse reactions (at least 20% in either treatment phase) are: injection site reactions, arthralgia, hypersensitivity reactions, headache, generalized skin reactions lasting at least 14 days, nausea, abdominal pain, vomiting, cough, oropharyngeal pain, pruritus, diarrhea, nasal congestion, fatigue, dizziness, and anxiety. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact BioMarin Pharmaceutical Inc. at 1-866-906-6100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Effect of Palynziq on Other PEGylated Products: Monitor for hypersensitivity reactions, including anaphylaxis, with concomitant treatment. (7.1)
USE IN SPECIFIC POPULATIONS
Pregnancy: May cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2020
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF ANAPHYLAXIS
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Blood Phenylalanine Monitoring and Diet
2.3 Premedication
2.4 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis
5.2 Palynziq REMS Program
5.3 Other Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 Effect of Palynziq on Other PEGylated Products
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF ANAPHYLAXIS
-
Anaphylaxis has been reported after administration of Palynziq and may occur at any time during treatment [see Warnings and Precautions (5.1)].
-
Administer the initial dose of Palynziq under the supervision of a healthcare provider equipped to manage anaphylaxis, and closely observe patients for at least 60 minutes following injection. Prior to self‑injection, confirm patient competency with self‑administration, and patient’s and observer’s (if applicable) ability to recognize signs and symptoms of anaphylaxis and administer auto‑injectable epinephrine, if needed [see Dosage and Administration (2.4)].
-
Consider having an adult observer for patients who may need assistance in recognizing and managing anaphylaxis during Palynziq treatment. If an adult observer is needed, the observer should be present during and for at least 60 minutes after Palynziq administration, should be able to administer auto‑injectable epinephrine, and call for emergency medical support upon its use [see Warnings and Precautions (5.1)].
-
Prescribe auto‑injectable epinephrine to all patients treated with Palynziq. Prior to the first dose, instruct the patient and observer (if applicable) how to recognize the signs and symptoms of anaphylaxis, how to properly administer auto‑injectable epinephrine, and to seek immediate medical care upon its use. Instruct patients to carry auto‑injectable epinephrine with them at all times during treatment with Palynziq [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
-
Consider the risks and benefits of readministering Palynziq following an episode of anaphylaxis. If the decision is made to readminister Palynziq, readminister the first dose under the supervision of a healthcare provider equipped to manage anaphylaxis and closely observe the patient for at least 60 minutes following the dose [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
-
Because of the risk of anaphylaxis, Palynziq is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Palynziq REMS [see Warnings and Precautions (5.2)].
-
-
1 INDICATIONS AND USAGEPalynziq is indicated to reduce blood phenylalanine concentrations in adult patients with phenylketonuria (PKU) who have uncontrolled blood phenylalanine concentrations greater than 600 micromol/L ...
Palynziq is indicated to reduce blood phenylalanine concentrations in adult patients with phenylketonuria (PKU) who have uncontrolled blood phenylalanine concentrations greater than 600 micromol/L on existing management.
Close -
2 DOSAGE AND ADMINISTRATION2.1 Dosage - Treatment with Palynziq should be managed by a healthcare provider experienced in the management of PKU. Obtain baseline blood phenylalanine concentration before initiating ...
2.1 Dosage
-
Treatment with Palynziq should be managed by a healthcare provider experienced in the management of PKU.
-
Obtain baseline blood phenylalanine concentration before initiating treatment.
Induction
The recommended initial induction dosage for Palynziq is 2.5 mg subcutaneously once weekly for 4 weeks. Administer the initial dose under the supervision of a healthcare provider [see Dosage and Administration (2.4)].
Titration
Titrate the Palynziq dosage in a step-wise manner, based on tolerability, over at least 5 weeks, to achieve a dosage of 20 mg subcutaneously once daily according to Table 1.
Maintenance
Therapeutic response may not be achieved until the patient is titrated to an effective maintenance dosage of Palynziq. Use the lowest effective and tolerated dosage of Palynziq.
Assess patient tolerability, blood phenylalanine concentrations, and dietary protein and phenylalanine intake throughout treatment.
Individualize the maintenance dosage to achieve blood phenylalanine control (blood phenylalanine concentrations less than or equal to 600 micromol/L), taking into account patient tolerability to Palynziq and dietary protein intake (see Table 1).
Maintain the Palynziq dosage at 20 mg once daily for at least 24 weeks. Consider increasing the Palynziq dosage to 40 mg once daily in patients who have been on 20 mg once daily continuously for at least 24 weeks without achieving blood phenylalanine control. Consider increasing the Palynziq dosage to a maximum of 60 mg once daily in patients who have been on 40 mg once daily continuously for at least 16 weeks without achieving blood phenylalanine control.
Discontinuation
Discontinue Palynziq in patients who have not achieved an adequate response after 16 weeks of continuous treatment with the maximum dosage of 60 mg once daily [see Clinical Studies (14)].
Table 1: Recommended Dosing Regimen - *
- Additional time may be required prior to each dosage escalation based on patient tolerability.
- †
- Individualize treatment to the lowest effective and tolerated dosage. Consider increasing to 40 mg once daily in patients who have not achieved a response with 20 mg once daily continuous treatment for at least 24 weeks. Consider increasing to a maximum of 60 mg once daily in patients who have not achieved a response with 40 mg once daily continuous treatment for at least 16 weeks [see Clinical Studies (14)].
- ‡
- Discontinue Palynziq in patients who have not achieved an adequate response after 16 weeks of continuous treatment at the maximum dosage of 60 mg once daily.
Treatment Palynziq Dosage Duration* Induction 2.5 mg once weekly 4 weeks Titration 2.5 mg twice weekly 1 week 10 mg once weekly 1 week 10 mg twice weekly 1 week 10 mg four times per week 1 week 10 mg once daily 1 week Maintenance†
20 mg once daily 24 weeks 40 mg once daily 16 weeks Maximum‡ 60 mg once daily 16 weeks Dose Reduction for Low Phenylalanine Concentrations
During titration and maintenance of Palynziq treatment, patients may experience blood phenylalanine concentrations below 30 micromol/L. For blood phenylalanine concentrations below 30 micromol/L, the dosage of Palynziq may be reduced and/or dietary protein and phenylalanine intake may be modified to maintain blood phenylalanine concentrations within a clinically acceptable range and above 30 micromol/L [see Dosage and Administration (2.2)].
Readministration Following Anaphylaxis
If the decision is made to readminister Palynziq after an anaphylaxis episode, administer the first dose following the anaphylaxis episode under the supervision of a healthcare provider equipped to manage anaphylaxis and closely observe the patient for at least 60 minutes following the dose. Subsequent dose titration should be based on patient tolerability and therapeutic response [see Warnings and Precautions (5.1)].
Missed Dose
If a dose is missed, instruct patients to take their next dose as scheduled and to not take two doses of Palynziq to make up for the missed dose.
2.2 Blood Phenylalanine Monitoring and Diet
After initiating treatment with Palynziq, obtain blood phenylalanine concentrations every 4 weeks until a maintenance dosage is established. After a maintenance dosage is established, periodic blood phenylalanine monitoring is recommended to assess blood phenylalanine control.
Monitor patients’ dietary protein and phenylalanine intake throughout treatment with Palynziq and counsel them on how to adjust their dietary intake, as needed, based on blood phenylalanine concentrations.
2.3 Premedication
For hypersensitivity reactions, consider premedication with an H1‑receptor antagonist, H2‑receptor antagonist, and/or antipyretic prior to Palynziq administration based upon individual patient tolerability [see Warnings and Precautions (5.1, 5.3)].
Close2.4 Administration Instructions
- Each prefilled syringe of Palynziq is intended for use as a single subcutaneous injection.
- Inspect Palynziq visually for particulate matter and discoloration prior to administration. Palynziq is a clear to slightly opalescent, colorless to pale yellow solution. Discard if discolored, cloudy, or if particulate matter is present.
- Prior to first dose of Palynziq, prescribe auto-injectable epinephrine, and instruct the patient and observer (if applicable) on how to recognize the signs and symptoms of anaphylaxis, how to properly administer auto-injectable epinephrine, and to seek immediate medical care upon its use.
- Perform initial administration(s) and/or readministration after an anaphylaxis episode under the supervision of a healthcare provider equipped to manage anaphylaxis, and closely observe patients for at least 60 minutes following injection [see Warnings and Precautions (5.1)]. Prior to self-injection, confirm patient competency with self‑administration.
- Consider having an adult observer for patients who may need assistance in recognizing and managing anaphylaxis during Palynziq treatment. If an adult observer is needed, the observer should be present during and for at least 60 minutes after each Palynziq administration, should be able to administer auto-injectable epinephrine, and to call for emergency medical support upon its use [see Warnings and Precautions (5.1)].
- The recommended injection sites for Palynziq are: the front middle of thighs and the abdomen at least 2 inches (five centimeters) away from the navel. If a caregiver is giving the injection, the top of buttocks and the back of the upper arms are also appropriate injection sites.
- Do not inject Palynziq into moles, scars, birthmarks, bruises, rashes, or areas where the skin is hard, tender, red, damaged, burned, inflamed, or tattooed. Check the injection site for redness, swelling, or tenderness.
- Rotate sites for subcutaneous injections of Palynziq. If more than one injection is needed for a single dose of Palynziq, the injection sites should be at least 2 inches away from each other. The second injection site can be on the same part of the body or a different part of the body.
-
-
3 DOSAGE FORMS AND STRENGTHSPalynziq is a clear to slightly opalescent, colorless to pale yellow solution available as follows: Injection: 2.5 mg/0.5 mL single-dose prefilled syringe - Injection: 10 mg/0.5 mL single-dose ...
Palynziq is a clear to slightly opalescent, colorless to pale yellow solution available as follows:
- Injection: 2.5 mg/0.5 mL single-dose prefilled syringe
- Injection: 10 mg/0.5 mL single-dose prefilled syringe
- Injection: 20 mg/mL single-dose prefilled syringe
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylaxis - In clinical trials of Palynziq with induction/titration/maintenance dosing, 29 out of 285 (10%) patients experienced a total of 42 anaphylaxis episodes [see Adverse Reactions ...
5.1 Anaphylaxis
In clinical trials of Palynziq with induction/titration/maintenance dosing, 29 out of 285 (10%) patients experienced a total of 42 anaphylaxis episodes [see Adverse Reactions (6.1, 6.2)]. The exposure-adjusted rate of anaphylaxis was highest during the induction and titration phases (0.25 episodes/person‑years; 5% of patients with at least one episode) and decreased in the maintenance phase (0.05 episodes/person‑years; 7% of patients with at least one episode). Signs and symptoms of anaphylaxis reported in clinical trials of Palynziq included syncope, hypotension, hypoxia, dyspnea, wheezing, chest discomfort/chest tightness, tachycardia, angioedema (swelling of face, lips, eyes, tongue), throat tightness, skin flushing, rash, urticaria, pruritus, and gastrointestinal symptoms (vomiting, nausea, diarrhea). In clinical trials of Palynziq, anaphylaxis generally occurred within 1 hour after injection (81%; 34/42 episodes); however, delayed episodes also occurred up to 48 hours after Palynziq administration. Most episodes of anaphylaxis occurred within the first year of dosing (69%, 29/42 episodes), but cases also occurred after one year of dosing and up to 1604 days (4.4 years) into treatment. Management of anaphylaxis in Palynziq clinical trials included: administration of auto-injectable epinephrine (48%; 20/42 episodes), corticosteroids (55%; 23/42 episodes), antihistamines (57%; 24/42 episodes), and/or oxygen (5%; 2/42 episodes). Twenty one out of the 29 (72%) patients who experienced anaphylaxis were rechallenged with Palynziq and 6 out of the 21 patients who were rechallenged (29%) had recurrence of anaphylaxis. All anaphylaxis episodes resolved without sequelae.
Consider having an adult observer for patients who may need assistance in recognizing and managing anaphylaxis during Palynziq treatment. If an adult observer is needed, the observer should be present during and for at least 60 minutes after Palynziq administration, should be able to administer auto‑injectable epinephrine, and to call for emergency medical support upon its use.
Anaphylaxis requires immediate treatment with auto-injectable epinephrine. Prescribe auto‑injectable epinephrine to all patients receiving Palynziq and instruct patients to carry auto‑injectable epinephrine with them at all times during Palynziq treatment. Prior to the first dose, instruct the patient and observer (if applicable) on how to recognize the signs and symptoms of anaphylaxis, how to properly administer auto‑injectable epinephrine, and to seek immediate medical care upon its use. Consider the risks associated with auto-injectable epinephrine use when prescribing Palynziq. Refer to the auto‑injectable epinephrine prescribing information for complete information.
Consider the risks and benefits of readministering Palynziq following an episode of anaphylaxis. If the decision is made to readminister Palynziq, administer the first dose under the supervision of a healthcare provider equipped to manage anaphylaxis and closely observe the patient for at least 60 minutes following the dose. Subsequent Palynziq dose titration should be based on patient tolerability and therapeutic response [see Dosage and Administration (2.4)].
Consider premedication with an H1-receptor antagonist, H2-receptor antagonist, and/or antipyretic prior to Palynziq administration based upon individual patient tolerability [see Dosage and Administration (2.3)].
Palynziq is available only through a restricted program under a REMS [see Warnings and Precautions (5.2)].
5.2 Palynziq REMS Program
Palynziq is available only through a restricted program under a REMS called the Palynziq REMS, because of the risk of anaphylaxis [see Warnings and Precautions (5.1)].
Notable requirements of the Palynziq REMS include the following:
- Prescribers must be certified with the program by enrolling in the program and completing training.
- Prescribers must prescribe auto‑injectable epinephrine with Palynziq.
- Pharmacies must be certified with the program and must dispense only to patients who are authorized to receive Palynziq.
- Patients must enroll in the program and be educated about the risk of anaphylaxis by a certified prescriber to ensure they understand the risks and benefits of treatment with Palynziq.
- Patients must have auto‑injectable epinephrine available at all times while taking Palynziq.
Further information, including a list of qualified pharmacies, is available at www.PALYNZIQREMS.com or by telephone 1‑855‑758‑REMS (1‑855‑758‑7367).
Close5.3 Other Hypersensitivity Reactions
Hypersensitivity reactions, other than anaphylaxis [see Warnings and Precautions (5.1), Adverse Reactions (6.1, 6.2)], have been reported in 204 out of 285 (72%) patients treated with Palynziq. The exposure adjusted rate of other hypersensitivity reactions was highest during the induction and titration phases (4.3 episodes/person-year; 50% of patients with at least one adverse reaction) and decreased in the maintenance phase (1.3 episodes/person-year; 61% of patients with at least one adverse reaction).
Consider premedication with an H1‑receptor antagonist, H2‑receptor antagonist, and/or antipyretic prior to Palynziq administration based upon individual patient tolerability [see Dosage and Administration (2.3)]. Management of hypersensitivity reactions should be based on the severity of the reaction, recurrence of the reaction, and the clinical judgement of the healthcare provider, and may include dosage adjustment, temporary drug interruption, or treatment with antihistamines, antipyretics, and/or corticosteroids.
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed below and in other sections of labeling: Anaphylaxis [see Warnings and Precautions (5.1)] Other Hypersensitivity Reactions [see Warnings and ...
The following serious adverse reactions are discussed below and in other sections of labeling:
- Anaphylaxis [see Warnings and Precautions (5.1)]
- Other Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect a total treatment exposure of 789 patient-years in 285 patients who received Palynziq in an induction/titration/maintenance regimen in clinical trials [see Clinical Studies (14)]. Of the 285 patients, 229 patients were exposed to Palynziq for 24 weeks, 209 patients were exposed for 1 year, 181 patients were exposed for 2 years, and 160 patients were exposed for 3 years or longer. The patient population was evenly distributed between male and female patients, the mean age was 29 years (range: 16 to 56 years), and 98% of patients were White.
The most common adverse reactions (at least 20% of patients in either treatment phase) were injection site reactions, arthralgia, hypersensitivity reactions, headache, generalized skin reactions lasting at least 14 days, nausea, abdominal pain, vomiting, cough, oropharyngeal pain, pruritus, diarrhea, nasal congestion, fatigue, dizziness, and anxiety.
Of the 285 patients exposed to Palynziq in an induction/titration/maintenance regimen in clinical trials, 44 (15%) patients discontinued treatment due to adverse reactions. The most common adverse reactions leading to treatment discontinuation were hypersensitivity reactions (6% of patients) including anaphylaxis (3% of patients), angioedema (1% of patients), arthralgia (4% of patients), generalized skin reactions lasting at least 14 days (2% of patients), and injection site reactions (1% of patients).
The most common adverse reactions leading to dosage reduction were arthralgia (15% of patients), hypersensitivity reactions (9% of patients), injection site reactions (4% of patients), alopecia (3% of patients), and generalized skin reactions lasting at least 14 days (2% of patients).
The most common adverse reactions leading to temporary drug interruption were hypersensitivity reactions (14% of patients), arthralgia (13% of patients), anaphylaxis (4% of patients), and injection site reactions (4% of patients).
Table 2 lists adverse reactions reported in at least 15% of patients treated with Palynziq in an induction/titration/maintenance dosage regimen in clinical trials, and illustrates the adverse reaction rates over time by treatment phase. Table 3 lists laboratory abnormalities reported in at least 10% of patients treated with Palynziq in an induction/titration/maintenance dosage regimen in clinical trials.
For these analyses, the induction/titration phase was defined as the time prior to reaching a stable dose (completing an 8‑week phase at the same dose level). Once a stable dosage was reached, patients were considered to be in the maintenance phase thereafter. Safety data for patients who reached the maintenance phase are included within either the induction/titration or maintenance phases depending on the onset date of the adverse reaction. Safety data for patients who did not reach the maintenance phase are included within the induction/titration phase. The maintenance phase includes data for patients who were previously on Palynziq and transitioned to placebo during the randomized withdrawal period of Study 302 [see Clinical Studies (14)].
Rates of adverse reactions (adjusted for duration of exposure) generally decreased over time and for some stayed relatively stable. In the maintenance phase, the rate of adverse reactions (adjusted for duration of exposure) in patients who reached the maintenance phase was comparable across dosages evaluated. The types and rate of adverse reactions reported during the maintenance phase in patients who received 20 mg once daily, 40 mg once daily, and 60 mg once daily were similar. During long-term treatment (greater than 36 months), the exposure-adjusted rates of adverse reactions decreased.
Rates of laboratory abnormalities (adjusted for duration of exposure) stayed relatively stable over time, except for complement C4 below lower limit of normal (LLN) and hs-CRP above 0.287 mg/dL over a 6 month period (both decreased over time) and hypophenylalaninemia (blood phenylalanine concentration below 30 micromol/L) on a single measurement (increased over time). There were no dose‑related trends in type or rate of laboratory abnormalities (adjusted for duration of exposure) reported during the maintenance phase in patients receiving 20 mg once daily, 40 mg once daily, or 60 mg once daily.
Table 2: Adverse Reactions* Reported in at least 15% of PKU Patients Treated with Palynziq in an Induction/Titration/Maintenance Regimen in Clinical Trials – Incidence and Exposure-Adjusted Rates - *
- ≥ 15% incidence in either treatment phase
- †
- N (%) = Number of patients with at least 1 Adverse Reaction (%); Rate = Exposure-Adjusted Rate of Adverse Reactions (Adverse Reactions/Person‑Years)
- ‡
-
Includes injection site: reaction, erythema, pruritus, pain, bruising, rash, swelling, urticaria, induration, hemorrhage, edema, mass, inflammation, nodule, discoloration, warmth, hematoma, irritation, vesicles, hypersensitivity, papule, discomfort, scar, paresthesia, hypertrophy, extravasation, dryness, scab
- §
-
Includes arthralgia, pain in extremity, back pain, musculoskeletal pain, neck pain
- ¶
- Includes rash, urticaria, anaphylaxis, rash generalized, hypersensitivity, rash erythematous, rash maculo-papular, rash pruritic, serum sickness, swelling face, dermatitis contact, swollen tongue, lip swelling, rash macular, pharyngeal edema, injection site hypersensitivity, eczema, drug eruption, dermatitis allergic, dermatitis, tongue edema, palatal edema, edema mouth, multiple allergies, lip edema, eye edema, exfoliative rash, drug hypersensitivity, dermatitis atopic, dermatitis acneiform, pruritus allergic, mouth swelling, implant site rash, gingival swelling, face edema, eyelid edema, eye swelling, dermatitis psoriasiform, dermatitis infected, conjunctivitis allergic, bronchospasm, angioedema, allergic sinusitis, allergic cough, eczema nummular, rhinitis allergic
- #
-
Includes headache, migraine, sinus headache
- Þ
- Includes pruritus, rash, urticaria, dry skin, rash erythematous, erythema, cellulitis, rash macular, pruritus generalized, petechiae, dermatitis allergic, skin infection, skin induration, rash maculo-papular, rash generalized, pharyngeal edema, macule, granulomatous dermatitis, exfoliative rash, drug eruption, dermatitis atopic, dermatitis, xanthogranuloma, skin plaque, skin mass, skin lesion, skin hypopigmentation, skin hyperpigmentation, skin exfoliation, septal panniculitis,scar, rash pruritic, rash papular, psoriatic arthropathy, pruritus allergic, papule, necrobiosis lipoidica diabeticorum, furuncle, eczema, ecchymosis, dermatitis psoriasiform, dermatitis infected, blister, eczema nummular, granuloma, infected dermal cyst, lipohypertrophy, psoriasis, skin irritation
- ß
- Includes abdominal pain, abdominal pain upper, abdominal discomfort
Treatment Phase
Treatment Duration
Induction/Titration Phase
(N = 285)
141 person-years
Mean: 188 days
Median: 116 days
Range: 1 to 2266 days
Maintenance Phase
(N = 225)
652 person-years
Mean: 1087 days
Median: 1158 days
Range: 5 to 2017 days
Adverse Reaction N (%)† Episodes
(Rate)†
N (%)† Episodes
(Rate)†
Injection site reactions‡ 252 (88%) 2965 (21) 166 (74%) 2169 (3.3) Arthralgia§ 211 (74%) 1049 (7.4) 154 (68%) 893 (1.4) Hypersensitivity reactions¶ 153 (54%) 634 (4.5) 145 (64%) 845 (1.3) Headache# 102 (36%) 214 (1.5) 126 (56%) 1049 (1.6) Generalized skin reaction lasting at least 14 daysÞ 61 (21%) 97 (0.7) 93 (41%) 186 (0.3) Nausea 52 (18%) 68 (0.5) 69 (31%) 141 (0.2) Abdominal painß 39 (14%) 54 (0.4) 67 (30%) 162 (0.3) Vomiting 36 (13%) 53 (0.4) 68 (30%) 139 (0.2) Cough 27 (9%) 33 (0.2) 67 (30%) 100 (0.2) Oropharyngeal pain 38 (13%) 45 (0.3) 65 (29%) 108 (0.2) Pruritus 58 (20%) 102 (0.7) 61 (27%) 424 (0.7) Diarrhea 26 (9%) 32 (0.2) 61 (27%) 116 (0.2) Nasal congestion 12 (4%) 16 (0.1) 61 (27%) 87 (0.1) Fatigue 37 (13%) 81 (0.6) 55 (24%) 110 (0.2) Dizziness 47 (16%) 65 (0.5) 48 (21%) 100 (0.2) Anxiety 14 (5%) 23 (0.2) 48 (21%) 100 (0.2) Alopecia 13 (5%) 14 (0.1) 43 (19%) 62 (0.1) Table 3: Laboratory Abnormalities Reported in at least 10% of PKU Patients Treated with Palynziq in an Induction/Titration/Maintenance Regimen in Clinical Trials – Incidence and Exposure-Adjusted Rates Treatment Phase
Treatment Duration
Induction/Titration Phase
(N = 285)
141 person‑years
Mean: 188 days
Median: 116 days
Range: 1 to 2266 days
Maintenance Phase
(N = 225)
652 person‑years
Mean: 1087 days
Median: 1158 days
Range: 5 to 2017 days
Laboratory Measurement N (%)* Episodes
(Rate)*
N (%)* Episodes
(Rate)*
Complement factor C3 below LLN 195 (68%) 453 (3.2) 188 (84%) 2259 (3.5) C-reactive protein (CRP) above ULN 182 (64%) 359 (2.5) 160 (71%) 1414 (2.2) Hypophenylalaninemia† on a single measurement 53 (19%) 216 (1.5) 147 (65%) 1553 (2.4) Complement factor C4 below LLN 177 (62%) 318 (2.3) 111 (49%) 714 (1.1) Hypophenylalaninemia† on 2 or more consecutive measurements 45 (16%) 62 (0.4) 111 (49%) 204 (0.3) Blood creatine phosphokinase (CPK) above ULN 50 (18%) 88 (0.6) 108 (48%) 377 (0.6) Hs-CRP above 0.287 mg/dL over a 6 month period 34 (12%) 34 (0.4) 36 (16%) 46 (0.1) LLN – lower limit of normal
ULN – upper limit of normal
Hs – high sensitivity
Description of Selected Adverse Reactions
Arthralgia
In clinical trials, 245 out of 285 (86%) patients experienced episodes consistent with arthralgia (includes back pain, musculoskeletal pain, pain in extremity, and neck pain).
Arthralgia episodes were more frequent during the induction/titration phase (7.4 episodes/patient-year) and decreased over time (1.4 episodes/patient-year in the maintenance phase).
Forty-four out of 285 (15%) patients had one episode of arthralgia, 32 (11%) patients had 2 episodes of arthralgia, 18 (6%) had 3 episodes of arthralgia, and 146 (51%) had 4 or more episodes of arthralgia. Arthralgia occurred as early as after the first dose of Palynziq and occurred at any time during treatment. The mean duration of arthralgia was 16 days (median: 3 days, range: 1 to 936 days), and 19% of arthralgia episodes had a duration of at least 14 days. Severe arthralgia (severe pain limiting self-care activities of daily living) was reported by 11 (4%) patients. In addition to arthralgia, other joint-related signs and symptoms reported were: joint swelling (24 patients; 8%), joint stiffness (22 patients; 8%), and musculoskeletal stiffness (20 patients; 7%). Arthralgia episodes were managed with medications (e.g., nonsteroidal anti-inflammatory drugs, glucocorticoids, and acetaminophen), Palynziq dosage reduction (4% of episodes), Palynziq interruption (4% of episodes), or Palynziq withdrawal (0.6% of episodes). 97% of arthralgia episodes were reported as resolved at the time of last observation (up to 77 months of follow‑up).
Injection Site Reactions
Injection site reactions were reported as early as after the first dose of Palynziq and occurred at any time during treatment. Injection site reactions were more frequent during the induction/titration phase (21 episodes/patient-years) and decreased over time (3 episodes/patient‑years in the maintenance phase). The mean duration of injection site reaction was 10 days (median: 2 days, range: 1 to 1612 days), and 8% of injection site reactions had a duration of at least 14 days. 99% of injection site reactions were reported as resolved at the time of last observation (up to 77 months of follow‑up).
Three injection site reactions consistent with granulomatous skin lesions were reported (each reaction occurring in one patient): granulomatous dermatitis (occurred after 464 days of Palynziq treatment and lasted 16 days), xanthogranuloma (occurred after 378 days of Palynziq treatment and lasted 638 days) was treated with a topical antihistamine, corticosteroid, and Palynziq treatment was discontinued, and necrobiosis lipoidica diabeticorum (occurred after 281 days of Palynziq treatment and lasted 281 days). Necrobiosis lipoidica diabeticorum was treated with steroid injections and complicated by Pseudomonas infection. All three injection site reactions resolved.
One patient reported soft tissue infection (occurred after 196 days of Palynziq treatment and lasted 8 days) associated with mesenteric panniculitis treated with antibiotics, which resulted in treatment discontinuation.
Generalized Skin Reactions (not limited to the injection site) Lasting at Least 14 Days
In clinical trials, 134 out of 285 (47%) patients treated with Palynziq experienced generalized skin reactions (not limited to the injection site) lasting at least 14 days. Mean duration of these reactions was 63 days (median: 37 days; range: 14 to 638 days). Generalized skin reactions were more frequent during the induction/titration phase (0.7 episodes/patient‑years), and decreased over time (0.3 episodes/patient-years in the maintenance phase).
The mean time from first dose of Palynziq to onset of skin reactions was 373 days (median: 213 days; range: 2 to 1970 days). 5% of these reactions persisted at least 180 days, and 86% of these reactions were reported as resolved at the time of last observation (up to 77 months of follow‑up).
Angioedema
In clinical trials, 22 out of 285 (8%) patients experienced 45 episodes of angioedema (symptoms included: pharyngeal edema, swollen tongue, lip swelling, mouth swelling, eyelid edema and face edema) occurring independent of anaphylaxis. Angioedema (included under Hypersensitivity in Table 2) was more frequent during the induction/titration phase (0.14 episodes/patient-year) and decreased over time (0.04 episodes/patient-year in the maintenance phase). Three patients discontinued treatment. All episodes resolved. Angioedema can present as a symptom of anaphylaxis [see Warnings and Precautions (5.1)].
Serum Sickness
In clinical trials, serum sickness was reported in 7 out of 285 (2%) patients. Serum sickness episodes were more frequent during the induction/titration phase (0.04 episodes/patient-year) and decreased over time (less than 0.01 episodes/patient-year during the maintenance phase). All serum sickness reactions resolved without sequelae (duration of serum sickness ranged from 1 to 8 days). Out of the 7 patients who experienced serum sickness, 5 patients continued treatment without a recurrence, and managed serum sickness with drug interruption, dosage reduction and/or concomitant medication. Two patients discontinued treatment.
Close6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Palynziq in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
All patients treated with Palynziq developed a sustained total anti-drug antibody (TAb) response with a majority of patients (91%; N = 235/258) developing that response by Week 4 of treatment. Mean TAb titers peaked 2 weeks after Palynziq initiation and remained elevated throughout treatment (greater than 3 years after treatment initiation). Anti-phenylalanine ammonia lyase (PAL) IgM antibodies were detected in a majority of patients (98%; N = 265/270) by 2 months after treatment initiation, with incidence declining over time to 67% at 36 months (N = 114/171). Anti‑PAL IgG antibodies were detected in almost all patients (N = 226/227) by 4 months after treatment initiation. Mean anti-PAL IgM and IgG titers peaked at approximately 3 and 6 months, respectively, after treatment initiation and remained elevated throughout treatment (greater than 3 years after treatment initiation). Drug-induced anti-PEG IgM and IgG antibodies were detected in the majority of patients (98%; N = 277/284 for IgM; and 278/284 for IgG) with mean titers for both peaking at 1 to 3 months after treatment initiation [see Drug Interactions (7.1)]. Neutralizing antibodies (NAb) capable of inhibiting PAL enzyme activity were detected on at least one measurement in the majority of patients (89%; N = 253/284) over time. Mean NAb titers peaked and reached a plateau at 16 to 20 weeks of treatment and then remained present throughout treatment (greater than 3 years after treatment initiation).
Twenty-seven of 29 patients who had anaphylaxis were tested for anti‑pegvaliase‑pqpz IgE antibodies, which recognize the PEGylated protein product. Of the 27 patients tested for anti‑pegvaliase‑pqpz IgE antibodies, 26 patients tested negative. The one patient who tested positive for anti‑pegvaliase‑pqpz IgE antibodies on the screening test did not have sufficient sample to confirm IgE positivity. This patient tested negative for anti‑pegvaliase‑pqpz IgE at routine visits prior to and after the anaphylaxis episode (not at times of anaphylaxis). Sixty‑seven of 285 patients in clinical trials were tested for both anti‑PAL IgE antibodies, which recognize the recombinant PAL protein, and for anti‑pegvaliase‑pqpz IgE antibodies during routine study visits (not at times of anaphylaxis episodes) or during additional visits for hypersensitivity reactions. Of those 67 patients, 5 (8%) tested positive at least once for anti‑PAL IgE antibodies but negative for anti‑pegvaliase‑pqpz IgE antibodies.
The highest frequency of hypersensitivity reactions (consistent with a Type III immune complex-mediated hypersensitivity mechanism) occurred within the first 6 months of Palynziq treatment when the mean circulating immune complex (CIC) concentrations were at their highest and mean complement C3 and C4 concentrations were at their lowest. Mean CIC concentrations decreased and complement levels increased over time as the exposure-adjusted rate of hypersensitivity reactions decreased. IgG and IgM CIC concentrations were above the upper limit of normal in 63% (N = 164/259) and 42% of patients (N = 109/259), respectively, at 12 weeks of Palynziq treatment and returned towards baseline with long-term treatment (greater than 3 years after treatment initiation). 61% of patients (N = 110/180) had complement C3 concentrations less than lower limit of normal (LLN) at 6 months after treatment initiation and 38% of patients (N = 94/248) had complement C4 concentrations less than LLN at 3 months after treatment initiation. The incidence of low complement C3 and C4 concentrations decreased over time, but approximately 35% (N = 34/96) and 12% (N = 11/96) of patients had low C3 and C4 concentrations, respectively, at 36 months after treatment initiation.
Higher antibody responses for all antibody analytes, including NAb, were associated with lower mean trough pegvaliase‑pqpz concentrations and with higher blood phenylalanine concentrations. Hypersensitivity reactions occurred more frequently in patients with higher antibody titers for some but not all antibody analytes. Patients with higher mean change in IgG CIC concentrations from pre-treatment baseline tended to have higher discontinuation rates than patients with lower mean change in IgG CIC concentrations. Mean antibody titers for anti‑PAL IgG and IgM, TAb, and NAb remained relatively stable with long-term treatment.
-
7 DRUG INTERACTIONS7.1 Effect of Palynziq on Other PEGylated Products - In a single dose study of Palynziq in adult patients with PKU, two patients receiving concomitant injections of medroxyprogesterone acetate ...Close
7.1 Effect of Palynziq on Other PEGylated Products
In a single dose study of Palynziq in adult patients with PKU, two patients receiving concomitant injections of medroxyprogesterone acetate suspension (a formulation containing PEG 3350) experienced a hypersensitivity reaction. One of the two patients experienced a hypersensitivity reaction on day 15 after a single Palynziq dosage of 0.67 mg within 15 minutes following medroxyprogesterone acetate injectable suspension, and subsequently experienced anaphylaxis on day 89 within 30 minutes after the next dose of medroxyprogesterone acetate injectable suspension. The other patient experienced a hypersensitivity reaction on day 40 after a single Palynziq dosage of 0.08 mg within 10 minutes following medroxyprogesterone acetate injectable suspension. Both patients had high anti‑PEG IgG antibody titers at or around the time of the hypersensitivity reactions.
In Palynziq clinical trials, the majority of patients developed anti‑PEG IgM and IgG antibodies after treatment with Palynziq [see Adverse Reactions (6.2)]. The clinical effects of concomitant treatment with different PEGylated products is unknown. Monitor patients treated with Palynziq and concomitantly with other PEGylated products for hypersensitivity reactions including anaphylaxis.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in studies of pregnant animals without PKU treated with pegvaliase-pqpz, Palynziq may cause fetal harm when administered to a pregnant woman ...
8.1 Pregnancy
Risk Summary
Based on findings in studies of pregnant animals without PKU treated with pegvaliase-pqpz, Palynziq may cause fetal harm when administered to a pregnant woman. Limited available data with pegvaliase-pqpz use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. There are risks to the fetus associated with poorly controlled phenylalanine concentrations in women with PKU during pregnancy including increased risk for miscarriage, major birth defects (including microcephaly, major cardiac malformations), intrauterine fetal growth retardation, and future intellectual disability with low IQ; therefore, phenylalanine concentrations should be closely monitored in women with PKU during pregnancy (see Clinical Considerations and Data). Advise pregnant women of the potential risks to the fetus.
A reproduction study in pregnant rabbits treated with pegvaliase‑pqpz demonstrated a high incidence of fetal malformations throughout the skeletal system, and in kidneys, lungs, and eyes. Embryo‑fetal toxicity (increased resorptions and reduced fetal weight) was also observed. These effects occurred at 5 times the maximum recommended daily dose and were associated with strong signs of maternal toxicity, including marked reductions in weight gain and food consumption, and death. A reproduction study in pregnant rats treated with pegvaliase‑pqpz demonstrated an increase in skeletal variations, with no malformations observed. The effects in rats occurred at 2.8 times the maximum recommended daily dose. In a pre-/post-natal development study in rats, pegvaliase‑pqpz produced reduced survival of offspring during lactation, decreases in pup weight and litter size, and delayed sexual maturation of offspring when administered daily at 13 times the maximum recommended daily dose. The effects on rat embryo‑fetal and post-natal development were also associated with maternal toxicity.
All pregnancies have a background risk of major birth defects, pregnancy loss, or other adverse pregnancy outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The estimated background risk of major birth defects and miscarriage in pregnant women with PKU who maintain blood phenylalanine concentrations greater than 600 micromol/L during pregnancy is greater than the corresponding background risk for pregnant women without PKU.
There is a pregnancy surveillance program for Palynziq. If Palynziq is administered during pregnancy, or if a patient becomes pregnant while receiving Palynziq or within one month following the last dose of Palynziq, healthcare providers should report Palynziq exposure by calling 1-866-906-6100.
Clinical Considerations
Disease-Associated Maternal and/or Embryo-Fetal Risk
Uncontrolled blood phenylalanine concentrations before and during pregnancy are associated with an increased risk of adverse pregnancy outcomes and fetal adverse effects. To reduce the risk of hyperphenylalaninemia-induced fetal adverse effects, blood phenylalanine concentrations should be maintained between 120 and 360 micromol/L during pregnancy and during the 3 months before conception [see Dosage and Administration (2.2)].
Dose Adjustments During Pregnancy and the Postpartum Period
Phenylalanine concentrations below 30 micromol/L in pregnant women with PKU treated with Palynziq may be associated with adverse fetal outcomes. Monitor blood phenylalanine concentrations during pregnancy and adjust the dosage of Palynziq or modify dietary protein and phenylalanine intake to avoid blood phenylalanine concentrations below 30 micromol/L [see Dosage and Administration (2.2)].
Data
Human Data
Uncontrolled Maternal PKU: Available data from the Maternal Phenylketonuria Collaborative Study on 468 pregnancies and 331 live births in pregnant women with PKU demonstrated that uncontrolled phenylalanine concentrations above 600 micromol/L are associated with an increased risk for miscarriage, major birth defects (including microcephaly, major cardiac malformations), intrauterine fetal growth retardation, and future intellectual disability with low IQ.
Limited data from case reports of Palynziq use in pregnant women are insufficient to determine a drug‑associated risk of adverse developmental outcomes.
Animal Data
All developmental toxicity studies were conducted in animals (rats and rabbits) without PKU, in which treatment with pegvaliase-pqpz produced a dose-dependent reduction in maternal blood phenylalanine concentrations. At doses that produced maternal toxicity and/or effects on embryo-fetal development, the maternal plasma phenylalanine concentrations were markedly reduced compared to the control group. The contribution of maternal phenylalanine depletion to the incidence of embryo‑fetal developmental effects was not evaluated.
Subcutaneous administration of 5 mg/kg/day pegvaliase‑pqpz (5 times the maximum recommended daily dose based on bodyweight [mg/kg]) in pregnant rabbits during the period of organogenesis produced embryo‑lethality (increased resorptions), marked reduction in fetal weight, and fetal malformations. The malformations included multiple external abnormalities of the head, body and limbs, multiple soft tissue malformations (reduced size or absence of kidneys, diaphragmatic hernia, corneal opacity, discoloration or reduced size of eyes, and reduced size of lungs) and multiple skeletal malformations of the craniofacial bones, vertebrae, sternebrae, ribs, pelvis, limbs, and digits. An increase in variations and delayed ossification was also observed in all skeletal regions. The adverse developmental effects were associated with maternal toxicity, as indicated by marked impairment of weight gain and food consumption. Deaths associated with weight loss and abortion occurred in 8% of the pregnant rabbits treated with 5 mg/kg/day pegvaliase‑pqpz.
Subcutaneous administration of 2 mg/kg/day pegvaliase‑pqpz (2 times the maximum recommended daily dose based on bodyweight [mg/kg]) in pregnant rabbits had no adverse effects on embryo-fetal development. Systemic exposure to pegvaliase‑pqpz was detected in fetuses from rabbits treated with 2 or 5 mg/kg/day.
Pegvaliase‑pqpz increased fetal alterations when administered daily in pregnant rats at doses of 8 mg/kg subcutaneously and higher (2.8 times the human steady-state area under the curve [AUC] at the maximum recommended daily dose) during a 28‑day premating period, mating, and through the period of organogenesis. The fetal alterations were limited to skeletal variations such as cervical ribs, bifid centra of lumbar and thoracic vertebrae, and incomplete ossification of squamosal bones, frontal bones, lumbar vertebra arch, and ribs. Daily administration of 20 mg/kg subcutaneously (13 times the human steady-state AUC at the recommended maximum daily dose) to pregnant rats produced reductions in litter sizes and fetal weights, which was associated with maternal toxicity (decreased body weight, ovarian weight, and food consumption). The decrease in litter sizes at 20 mg/kg subcutaneously was secondary to reductions in corpora lutea and implantations. Systemic exposure to pegvaliase‑pqpz was detected in fetuses from rats treated with 20 mg/kg of pegvaliase‑pqpz (13 times the human steady-state AUC at the recommended maximum daily dose). Subcutaneous administration of 2 mg/kg/day pegvaliase‑pqpz (less than the human steady state AUC at the maximum recommended daily dose) in pregnant rats had no adverse effects on embryo‑fetal development.
Pegvaliase-pqpz decreased pup weight, litter size, and survival of offspring during lactation, and delayed sexual maturation of offspring when administered daily in rats at 20 mg/kg subcutaneously (13 times the human steady-state AUC at the recommended maximum daily dose), with dosing starting before mating and continuing through lactation. The effects in offspring were associated with maternal toxicity. No effects in offspring were observed at 8 mg/kg/day subcutaneously (2.8 times the human steady-state AUC at the recommended maximum daily dose). A follow-up study of the same design evaluated additional parameters of physical and neurobehavioral development in offsprings; No effects of pegvaliase‑pqpz were noted at the maternal NOAEL dose of 8 mg/kg/day.
8.2 Lactation
Risk Summary
There are no data on the presence of pegvaliase-pqpz in human milk, the effects on the breastfed infant, or the effects on milk production. A pre-/post-natal study in rats showed that pegvaliase-pqpz is present in rat milk and that administration of pegvaliase-pqpz during lactation decreased pup weight and survival [see Use in Specific Populations (8.1)]. However, systemic absorption of pegvaliase-pqpz was not detected in the rat pups. Palynziq may cause low phenylalanine concentrations in human milk. The developmental and health benefits of breastfeeding should be considered along with the clinical need for Palynziq treatment and any potential adverse effect on the breastfed infant from Palynziq or from the underlying condition (see Clinical Considerations).
Clinical Considerations
Monitor blood phenylalanine concentrations in breastfeeding women treated with Palynziq.
Close8.4 Pediatric Use
The safety and effectiveness of Palynziq in pediatric patients have not been established.
-
11 DESCRIPTIONPegvaliase-pqpz is a phenylalanine-metabolizing enzyme that is composed of recombinant phenylalanine ammonia lyase (rAvPAL) conjugated to N-hydroxysuccinimide (NHS)-methoxypolyethylene glycol ...
Pegvaliase-pqpz is a phenylalanine-metabolizing enzyme that is composed of recombinant phenylalanine ammonia lyase (rAvPAL) conjugated to N-hydroxysuccinimide (NHS)-methoxypolyethylene glycol (PEG). rAvPAL is manufactured in Escherichia coli bacteria transformed with a plasmid containing the phenylalanine ammonia lyase (PAL) gene derived from Anabaena variabilis. During the rAvPAL manufacturing process, fermentation is carried out in nutrient medium containing the antibiotic kanamycin. However, kanamycin is cleared in the manufacturing process and is not detectable in the final product. rAvPAL is a homotetrameric protein with a molecular weight of 62 kD per monomer. To produce pegvaliase-pqpz, an average of nine (9) 20 kD PEG molecules are covalently bound (or conjugated) to each monomer of rAvPAL. The total molecular weight of pegvaliase‑pqpz (rAvPAL‑PEG) is approximately 1000 kD.
Palynziq (pegvaliase‑pqpz) injection, intended for subcutaneous injection, is a clear to slightly opalescent, colorless to pale yellow, sterile, preservative-free solution and is formulated at pH 6.6 to 7.4.
Palynziq is provided in a single-dose prefilled syringe and is available in three dosage strengths: 2.5 mg/0.5 mL, 10 mg/0.5 mL, and 20 mg/mL. Palynziq contents for each dosage strength are summarized in Table 4.
Table 4: Contents of Palynziq Strength
Total Contents per Prefilled Syringe
Palynziq 2.5 mg/0.5 mL
prefilled syringe
2.5 mg pegvaliase-pqpz (expressed as the amount of rAvPAL conjugated to 7.25 mg of 20 kD PEG in 0.5 mL Water for Injection, USP) and contains the following inactive ingredients: sodium chloride (for tonicity adjustment), trans-cinnamic acid (0.07 mg), and tromethamine and tromethamine hydrochloride (for pH adjustment).
Palynziq 10 mg/0.5 mL
prefilled syringe
10 mg pegvaliase-pqpz (expressed as the amount of rAvPAL conjugated to 29 mg of 20 kD PEG in 0.5 mL Water for Injection, USP) and contains the following inactive ingredients: sodium chloride (for tonicity adjustment), trans-cinnamic acid (0.07 mg), and tromethamine and tromethamine hydrochloride (for pH adjustment).
Palynziq 20 mg/mL
prefilled syringe
20 mg pegvaliase-pqpz (expressed as the amount of rAvPAL conjugated to 58 mg of 20 kD PEG in 1 mL Water for Injection, USP) and contains the following inactive ingredients: sodium chloride (for tonicity adjustment), trans-cinnamic acid (0.15 mg), and tromethamine and tromethamine hydrochloride (for pH adjustment).
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Pegvaliase-pqpz is a PEGylated phenylalanine ammonia lyase (PAL) enzyme that converts phenylalanine to ammonia and trans‑cinnamic acid. It substitutes for the deficient ...
12.1 Mechanism of Action
Pegvaliase-pqpz is a PEGylated phenylalanine ammonia lyase (PAL) enzyme that converts phenylalanine to ammonia and trans‑cinnamic acid. It substitutes for the deficient phenylalanine hydroxylase (PAH) enzyme activity in patients with PKU and reduces blood phenylalanine concentrations.
12.2 Pharmacodynamics
Palynziq treatment of adult patients with PKU resulted in the reduction of blood phenylalanine concentrations from pre-treatment baseline [see Clinical Studies (14)]. The reduction of blood phenylalanine concentrations diminished with decreased pegvaliase‑pqpz plasma concentrations.
Close12.3 Pharmacokinetics
The pharmacokinetics of pegvaliase‑pqpz exhibit high inter-patient and intra-patient variability due to the heterogeneity of the immune response in adult patients with PKU. Higher antibody titers correlated with higher apparent clearance of pegvaliase‑pqpz. In the first eight weeks of induction/titration treatment, plasma pegvaliase‑pqpz concentrations were low to not measurable. At steady state during maintenance treatment with Palynziq 20 mg, 40 mg, and 60 mg subcutaneously once daily, the mean ± SD (range) plasma trough pegvaliase‑pqpz concentrations were: 11.2 ± 9.0 (0.21 to 29.6) mg/L, 10.4 ± 12.7 (0.18 to 43.1) mg/L and 4.8 ± 10.7 (0 to 39.7) mg/L, respectively. The following pharmacokinetic parameters were observed in adult patients with PKU treated with Palynziq at maintenance dosages of 20 mg once daily and 40 mg once daily.
Absorption
The median Tmax was approximately 8 hours. The mean ± SD (range) peak concentration (Cmax) at steady state was: 14.0 ± 16.3 (0.26 to 68.5) mg/L and 16.7 ± 19.5 (0.24 to 63.8) mg/L, respectively.
Distribution
The mean ± SD (range) apparent volume of distribution was 26.4 ± 64.8 (1.8 to 241) L and 22.2 ± 19.7 (3.1 to 49.5) L, respectively.
Elimination
The mean ± SD (range) apparent clearance at steady state was 0.39 ± 0.87 (0.018 to 3.66) L/h and 1.25 ± 2.46 (0.034 to 8.88) L/h, respectively. The mean ± SD (range) half-life was 47 ± 42 (14 to 132) hours and 60 ± 45 (14 to 127) hours, respectively.
Metabolism
The metabolism of phenylalanine ammonia lyase is expected to occur via catabolic pathways and be degraded into small peptides and amino acids.
Excretion
The route of elimination of pegvaliase‑pqpz has not been studied in humans.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity and genotoxicity studies have not been performed with pegvaliase‑pqpz. Based on its mechanism of action ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and genotoxicity studies have not been performed with pegvaliase‑pqpz. Based on its mechanism of action, pegvaliase‑pqpz is not expected to be tumorigenic.
Pegvaliase-pqpz produced impaired fertility in female rats at 20 mg/kg/day subcutaneously (13 times the human steady-state AUC at the maximum recommended daily dose), as indicated by decreases in corpora lutea, implantations, and litter size. These effects were associated with maternal toxicity (decreased body weight, ovarian weight, and food consumption). No effects on mating or fertility were observed in female rats with 8 mg/kg/day subcutaneously (2.8 times the human steady-state AUC at the maximum recommended daily dose) or in male rats with 20 mg/kg/day subcutaneously.
Close13.2 Animal Toxicology and/or Pharmacology
In rats without PKU treated with pegvaliase-pqpz, dose-dependent vacuolation in multiple organs and tissues was observed in the 4‑ and 26‑week repeat dose toxicity studies at doses of 8 mg/kg subcutaneously or greater administered twice weekly (less than the human steady state AUC at the maximum recommended daily dose). Vacuolation occurred in renal tubule cells and in histiocytic cells of the liver, spleen, testes, adrenal cortex, mesenteric lymph node, and mandibular lymph node. Vacuolation in histiocytes of the affected organs and tissues persisted after cessation of treatment. The vacuolation observed in these studies was not associated with organ‑related toxicities as determined by clinical chemistry/urinalysis and histopathological examination. The clinical significance of these findings and functional consequences are unknown.
In the 39‑week repeat dose toxicity study in monkeys, pegvaliase-pqpz 3 mg/kg subcutaneously twice weekly (2 times the human steady state AUC at the maximum recommended daily dose) produced systemic arteritis involving small arteries and arterioles in a wide range of organs and tissues (kidney, urinary bladder, pancreas, gallbladder, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, lung, heart, sciatic nerve, lacrimal gland, mandibular lymph node, epididymis, seminal vesicle, ovary, uterus, cervix, and vagina) and in subcutaneous injection sites. Arteritis was likely due to the immune-mediated response (e.g., immune complex deposition in blood vessels) associated with chronic administration of a foreign protein to the animals. The incidence and severity of systemic arteritis was dose-dependent. The vascular inflammation observed in this study was not associated with organ related toxicities as determined by clinical pathology parameters (hematology, clinical chemistry, and urinalysis) and histopathological examination.
Studies of longer duration in rats and monkeys treated with pegvaliase‑pqpz have not been conducted.
-
14 CLINICAL STUDIESStudy 301: Induction/Titration/Maintenance Treatment - Study 165‑301 (referred to as Study 301, NCT01819727) was an open-label randomized, multi-center study of adults with PKU to assess safety ...
Study 301: Induction/Titration/Maintenance Treatment
Study 165‑301 (referred to as Study 301, NCT01819727) was an open-label randomized, multi-center study of adults with PKU to assess safety and tolerability of self-administered Palynziq in an induction/titration/maintenance regimen with a target maintenance dose of 20 mg subcutaneously once daily or 40 mg subcutaneously once daily. At Palynziq treatment initiation, 253 patients demonstrated inadequate blood phenylalanine control (blood phenylalanine concentration greater than 600 micromol/L) on existing management, and 8 patients had blood phenylalanine concentrations less than or equal to 600 micromol/L. Existing management options included prior or current restriction of dietary phenylalanine and protein intake, and/or prior treatment with sapropterin dihydrochloride. Patients previously treated with sapropterin dihydrochloride were required to discontinue use at least 14 days prior to the first dose.
The 261 enrolled patients were aged 16 to 55 years (mean: 29 years) and had a baseline mean (range) blood phenylalanine of 1,233 (285, 2330) micromol/L. One hundred forty nine out of 261 (57%) patients were taking medical food at baseline and 41 out of 261 patients (16%) were on a protein-restricted diet at baseline (defined as receiving greater than 75% of total protein intake from medical food). Patients were randomized (1:1) to one of two target maintenance dosage arms: 20 mg once daily or 40 mg once daily. Patients were titrated to reach their randomized target dosage of 20 mg once daily or 40 mg once daily. The duration of titration varied among patients and was based on patient tolerability. Of the 261 enrolled patients, 195 (75%) patients reached their randomized maintenance dosage (103 in the 20 mg once daily arm, 92 in the 40 mg once daily arm). Among the patients who reached their randomized maintenance dosage, patients in the 20 mg once daily randomized arm reached their maintenance dosage at a median time of 10 weeks (range: 9 to 29 weeks) and patients in the 40 mg once daily arm reached their maintenance dosage at a median time of 11 weeks (range: 10 to 33 weeks).
Of the 261 patients who enrolled in Study 301, 54 (21%) patients discontinued treatment during Study 301, 4 patients completed Study 301 and did not continue to Study 165‑302 (referred to as Study 302, NCT01889862), 152 patients continued to the eligibility period of Study 302, and 51 patients continued directly from Study 301 into the long‑term treatment period of Study 302.
Study 302: Efficacy Assessment
A total of 164 adult patients with PKU who were previously-treated with Palynziq (152 patients from Study 301 and 12 patients from other Palynziq trials) enrolled in Study 302 and continued treatment with Palynziq in Study 302 for up to 13 weeks to assess eligibility for randomized withdrawal period.
Randomized Withdrawal Period
Following this period of up to 13 weeks of additional Palynziq treatment in Study 302, eligibility for entry into the efficacy assessment period (randomized withdrawal period) was determined by whether a patient achieved at least a 20% reduction in blood phenylalanine concentration from pre-treatment baseline (when in previous studies). Eighty‑six out of 164 patients (52%) met this response target and continued into the randomized withdrawal period. In the double-blind, placebo-controlled, randomized withdrawal period, patients were randomized in a 2:1 ratio to either continue their maintenance Palynziq dosage or to receive matching placebo for a total of 8 weeks. The treatment difference in least squares (LS) mean change in blood phenylalanine concentration from the Study 302 randomized withdrawal baseline to randomized withdrawal Week 8 for each randomized study arm is shown in Table 5. Mean blood phenylalanine concentrations at pre-treatment baseline (Study 301 or other Palynziq trials) are also shown in Table 5. At Study 302 randomized withdrawal Week 8, Palynziq‑treated patients (20 mg once daily or 40 mg once daily) maintained their blood phenylalanine concentrations as compared to their randomized withdrawal baseline, whereas patients randomized to matching placebo (20 mg once daily or 40 mg once daily) returned to their pretreatment baseline blood phenylalanine concentrations (Figure 1).
Table 5: Primary Endpoint: LS Mean Change in Blood Phenylalanine Concentration from Randomized Withdrawal Baseline to Week 8 in Adult Patients with PKU – Efficacy Assessment in Study 302 - *
- Based on the mixed model repeated measures (MMRM) method, with treatment arm, visit, and treatment arm-by-visit interaction as factors adjusting for baseline blood phenylalanine concentration.
- †
- Patients who did not complete phenylalanine assessment within the window for Week 8 (Day 43 to 56) were excluded.
Randomized Study Arm Blood Phenylalanine Concentration (micromol/L) Mean (SD) LS Mean Change from Study 302 Randomized Withdrawal Baseline to Week 8 (95% CI)
Treatment Difference in LS Mean Change (95% CI) P‑value* Pre‑treatment Baseline Study 302 Randomized Withdrawal Baseline Study 302 Randomized Withdrawal Week 8 Palynziq 20 mg once daily
1450.2 (310.5)
n = 29
596.8 (582.8)
n = 29
553.0 (582.4)
n = 26†
-23.3
(-156.2, 109.7)
-973.0
(‑1204.2, ‑741.9)
p < 0.0001
Placebo 20 mg once daily
1459.1 (354.7)
n = 14
563.9 (504.6)
n = 14
1509.0 (372.6)
n = 13†
949.8
(760.4, 1139.1)
Palynziq 40 mg once daily
1185.8 (344.0)
n = 29
410.9 (440.0)
n = 29
566.3 (567.5)
n = 23†
76.3
(-60.2, 212.8)
-588.5 (-830.1, -346.9)
p < 0.0001
Placebo 40 mg once daily
1108.9 (266.8)
n = 14
508.2 (363.7)
n = 14
1164.4 (343.3)
n = 10†
664.8
(465.5, 864.1)
Figure 1: Observed Mean (SE) Blood Phenylalanine Concentrations Over Time in Adult Patients with PKU in Study 302
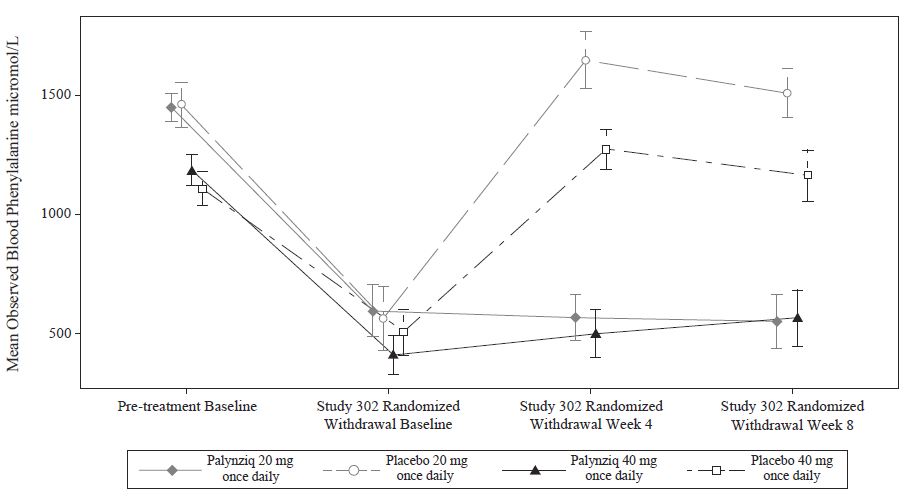
Study 301 and 302 Continuous Treatment
Of 118 patients from Study 301 with a pre‑treatment baseline blood phenylalanine concentration greater than 600 micromol/L who were randomized to and received at least one dose of 20 mg once daily Palynziq, 107 patients, 97 patients, 93 patients, 86 patients, and 77 patients were treated for at least 6 months, 12 months, 18 months, 24 months, and 36 months, respectively. During the continuous treatment period, patients were allowed to dose up to 60 mg once daily based on investigator discretion to achieve blood phenylalanine lowering (for example, to achieve blood phenylalanine concentrations between 120 and 360 micromol/L).
Of the 118 patients, a majority (91 patients, 77%) reached their first response, defined as a blood phenylalanine concentration less than or equal to 600 micromol/L, at a time point prior to 36 months. Of the 91 patients, 9 patients (10%) achieved their first response at a dose less than 20 mg once daily, 44 patients (48%) achieved their first response at a dose of 20 mg once daily, 26 patients (29%) achieved their first response at a dose of 40 mg once daily, and 12 patients (13%) achieved their first response at a dose of 60 mg once daily. Of the 44 patients that achieved their first response at a dose of 20 mg once daily, 36 (82%) achieved it by 24 weeks of treatment. Of the 26 patients that achieved their first response at a dose of 40 mg once daily, 18 (69%) achieved it by 16 weeks of treatment. Of the 12 patients that achieved their first response at a dose of 60 mg once daily, 8 (67%) achieved it by 16 weeks of treatment.
Of the 107 patients treated for at least 6 months, 28 (26%), 18 (17%), and 11 (10%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 6 months of treatment. Of the 97 patients treated for at least 12 months, 47 (48%), 41 (42%), and 31 (32%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 12 months of treatment. Of the 93 patients treated for at least 18 months, 64 (69%), 46 (49%), and 36 (39%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 18 months of treatment. Of the 86 patients treated for at least 24 months, 65 (76%), 57 (66%), and 43 (50%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 24 months of treatment. Of the 77 patients treated for at least 36 months, 58 (75%), 51 (66%), and 37 (48%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 36 months of treatment.
Close -
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Palynziq (pegvaliase-pqpz) injection is supplied as a preservative-free, sterile, clear to slightly opalescent, colorless to pale yellow solution. All dosage strengths of Palynziq ...
How Supplied
Palynziq (pegvaliase-pqpz) injection is supplied as a preservative-free, sterile, clear to slightly opalescent, colorless to pale yellow solution. All dosage strengths of Palynziq are provided in a 1 mL glass syringe with a 26 gauge, 0.5 inch needle.
Each carton contains 1 or 10 trays with single-dose prefilled syringe(s), Prescribing Information, Medication Guide, and Instructions for Use. The following packaging configurations are available.
Table 6: Palynziq Packaging Configurations Pegvaliase‑pqpz 2.5 mg/0.5 mL
1 syringe/carton
NDC 68135‑058‑90 Pegvaliase‑pqpz 10 mg/0.5 mL 1 syringe/carton
NDC 68135‑756‑20 Pegvaliase‑pqpz 20 mg/mL 1 syringe/carton
10 syringes/carton
NDC 68135‑673‑40
NDC 68135‑673‑45
Storage and Handling
- Store in refrigerator at 36°F to 46°F (2°C to 8°C) in its original carton to protect from light.
- Do not freeze or shake.
- For patients: If needed, store Palynziq in the original carton at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days. Record the date removed from refrigeration on the carton. Once stored at room temperature, do not return the product to the refrigerator.
- The shelf-life expires after storage at room temperature for 30 days, or after the expiration date on the product carton, whichever is earlier.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Anaphylaxis and Other Hypersensitivity Reactions - Advise patients that Palynziq may ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Anaphylaxis and Other Hypersensitivity Reactions
- Advise patients that Palynziq may cause hypersensitivity reactions, including anaphylaxis that can occur at any time. Instruct patients to recognize the signs and symptoms of anaphylaxis [see Warnings and Precautions (5.1, 5.3)].
- Instruct patients to carry auto-injectable epinephrine with them at all times during Palynziq treatment. Instruct the patient and observer (if applicable) on the appropriate use of auto‑injectable epinephrine for anaphylaxis [see Warnings and Precautions (5.1)].
- Instruct patients who experience anaphylaxis to seek immediate medical care, discontinue therapy, and resume treatment only at the instruction of a healthcare provider [see Warnings and Precautions (5.1)].
Palynziq REMS Program
Palynziq is available only through a restricted program called the Palynziq REMS [see Warnings and Precautions (5.2)]. Inform the patient of the following notable requirements:
- Patients must be enrolled in the Palynziq REMS.
- Patients must be educated about the risk of anaphylaxis by a certified prescriber to ensure they understand the risks and benefits of treatment with Palynziq.
- Patients must fill a prescription for auto-injectable epinephrine and carry it with them at all times.
- Patients will be given a Palynziq Patient Wallet Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the patient and observer (if applicable) to immediately seek medical care. Advise the patient to show the Palynziq Wallet Card to other treating healthcare providers.
Palynziq is available only from certified pharmacies participating in the program. Therefore, provide patients with the telephone number and website for information on how to obtain the product.
Administration
- Advise patients to monitor their dietary protein and phenylalanine intake throughout treatment with Palynziq, and adjust intake as directed by their healthcare provider based on blood phenylalanine concentrations [see Dosage and Administration (2.2)].
- Provide appropriate instruction for methods of self-injection, including careful review of the Palynziq Medication Guide and Instructions for Use. Instruct patients in the use of aseptic technique when administering Palynziq [see Dosage and Administration (2.4)].
- Inform patients that a healthcare provider will show them or their caregiver how to prepare to inject Palynziq before self-administering.
- Advise patients not to inject into moles, scars, birthmarks, bruises, rashes, or areas where the skin is hard, tender, red, damaged, burned, inflamed, or tattooed.
- Advise patients to rotate areas of injection with each dose. Advise patients to check the injection site for redness, swelling, and tenderness, and to contact their healthcare provider if they have a skin reaction and it does not clear up, or worsens.
- Advise patients to follow sharps disposal recommendations [see Instructions for Use] patients on safe disposal procedures.
- Advise patients that the shelf‑life expires after storage at room temperature for 30 days or after the expiration date on the product carton, whichever is earlier.
Pregnancy
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
- Advise women who are exposed to Palynziq during pregnancy or who become pregnant within one month following the last dose of Palynziq that there is a pregnancy surveillance program that monitors pregnancy outcomes. Encourage these patients to report their pregnancy to BioMarin (1‑866‑906‑6100) [see Use in Specific Populations (8.1)].
Manufactured by:
BioMarin Pharmaceutical Inc.
Novato, CA 94949
US License No. 1649
Close -
MEDICATION GUIDEMedication Guide - Palynziq® (Pal-lin-zeek) (pegvaliase-pqpz) Injection, for subcutaneous use - What is the most important information I should know about Palynziq? Palynziq can ...Close
Medication Guide
Palynziq® (Pal-lin-zeek)
(pegvaliase-pqpz)
Injection, for subcutaneous use
What is the most important information I should know about Palynziq? Palynziq can cause a severe allergic reaction (anaphylaxis) that may be life-threatening and can happen any time during treatment with Palynziq. Severe allergic reactions are a serious but common side effect of Palynziq.
- You will receive your first injection of Palynziq in a healthcare setting where you will be closely watched for at least 1 hour after your injection for a severe allergic reaction.
- If you have a severe allergic reaction during treatment with Palynziq, you will need to receive an auto-injection of epinephrine immediately and get emergency medical help right away.
- Your healthcare provider will decide if you (or your caregiver) are able to give the Palynziq injections, recognize the signs and symptoms of a severe allergic reaction, give an injection of epinephrine and call for emergency medical help, if needed.
- Your healthcare provider may recommend that an adult observer (or your caregiver) be with you when you give your Palynziq injection, and for at least 1 hour after your injection to watch you for signs and symptoms of a severe allergic reaction and, if needed, give you an injection of epinephrine and call for emergency medical help.
Stop injecting Palynziq and get emergency medical care right away if you have any of the following symptoms of a severe allergic reaction during treatment with Palynziq:
- fainting (passing out)
- dizziness or lightheadedness
- sudden confusion
- trouble breathing or wheezing
- chest discomfort or chest tightness
- fast heart rate
- swelling of your face, lips, eyes, or tongue
- throat tightness
- flushed skin
- skin rash, itching, or raised bumps on skin
- nausea, vomiting, or diarrhea
- losing control of urine or stools
- Your healthcare provider will prescribe an auto-injectable epinephrine for you and will teach you (or your caregiver) and your observer, if needed, when and how to use it if you have a severe allergic reaction. Keep the auto-injectable epinephrine with you at all times during treatment with Palynziq. Read the Patient Information that comes with the auto-injectable epinephrine that your healthcare provider prescribes for you for more information.
-
If you have a severe allergic reaction, do not continue to take Palynziq until you talk with your healthcare provider. Tell your healthcare provider that you had a severe allergic reaction. Your healthcare provider will tell you if you can continue treatment with Palynziq.
- Your healthcare provider may prescribe other medicines to take before your Palynziq injection that may help reduce the symptoms of an allergic reaction.
- If your healthcare provider decides that you can continue treatment with Palynziq after a severe allergic reaction, you will receive your next injection of Palynziq in a healthcare setting where you will be closely watched for at least 1 hour after your injection for a severe allergic reaction.
- Your healthcare provider will give you a Palynziq Patient Wallet Card that describes symptoms that you (or your caregiver), or your observer, should know that require you to get emergency medical care right away. Carry this card with you at all times during treatment with Palynziq. It is important to show your Palynziq Patient Wallet Card to any other healthcare provider who treats you.
Palynziq is only available through a restricted program called the Palynziq Risk Evaluation and Mitigation Strategy (REMS) Program. Before you can receive Palynziq, you must:
- enroll in this program.
- receive education about the risk of a severe allergic reaction (anaphylaxis) by a healthcare provider certified in the Palynziq REMS to be sure that you understand the risks and benefits of treatment with Palynziq.
- fill the prescription your healthcare provider gives you for the auto-injectable epinephrine and carry it with you at all times during treatment with Palynziq.
- carry the Palynziq Patient Wallet Card with you at all times.
Talk to your healthcare provider for more information about the Palynziq REMS and how to enroll.
What is Palynziq? Palynziq is a prescription medicine used to lower blood levels of phenylalanine in adults with PKU (phenylketonuria) who have uncontrolled blood phenylalanine levels above 600 micromol/L (10 mg/dL) on their current treatment.
It is not known if Palynziq is safe and effective in children.Before injecting Palynziq, tell your healthcare provider about all of your medical conditions, including if you:
- cannot or are not willing to use auto-injectable epinephrine to treat a severe allergic reaction.
- are pregnant or plan to become pregnant. Palynziq may harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you might be pregnant while taking Palynziq.
- If your phenylalanine levels are too high or too low during pregnancy, this may also affect your unborn baby. You and your healthcare provider can decide the best way for you to manage your blood phenylalanine levels and discuss the risks and benefits of taking Palynziq during pregnancy to you and your unborn baby. It is very important to keep your phenylalanine levels at the levels your healthcare provider recommends during pregnancy.
- Pregnancy Surveillance Program. There is a pregnancy surveillance program for females who take Palynziq during pregnancy, or who become pregnant while receiving Palynziq or within 1 month after their last dose of Palynziq. The purpose of this program is to collect information about the health of you and your baby while taking Palynziq. Talk to your healthcare provider about how you can take part in this program or call BioMarin at 1-866-906-6100.
- are breastfeeding or plan to breastfeed. It is not known if Palynziq passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Palynziq.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements.
How should I take Palynziq?
- Your healthcare provider will give you the Palynziq injection until they decide that you (or your caregiver) can give it. See “What is the most important information I should know about Palynziq?”
- Your healthcare provider may prescribe medicine for you to take before your Palynziq injection to help reduce the symptoms of an allergic reaction.
- If your healthcare provider decides that you (or your caregiver) can give your Palynziq injections, you (or your caregiver) will be shown how to prepare and give your Palynziq injections. See the “Instructions for Use” for detailed instructions on how to prepare and give an injection of Palynziq.
- Use Palynziq exactly as your healthcare provider tells you to. Your healthcare provider will tell you how much Palynziq to inject and when to inject it.
- Palynziq comes in prefilled syringes with 3 different strengths (2.5 mg, 10 mg, or 20 mg). You may need more than 1 Palynziq prefilled syringe for your prescribed dose.
- Monitor the amount of protein and phenylalanine that you eat or drink. Your healthcare provider may change the amount of protein and phenylalanine you should have in your diet during treatment with Palynziq, depending on the levels of phenylalanine in your blood. Follow your healthcare provider’s instructions about the amount of protein and phenylalanine you should have in your diet.
- If you miss a dose, take your next dose at the regular time. Do not take two doses of Palynziq to make up for a missed dose.
What are the possible side effects of Palynziq? Palynziq may cause serious side effects, including:
- See “What is the most important information I should know about Palynziq?”
-
Other allergic reactions to Palynziq can happen during treatment with Palynziq. Contact your healthcare provider right away if you have any of the following symptoms of an allergic reaction including: rash, itching, or swelling of the face, lips, eyes, or tongue. Your healthcare provider may change your dose of Palynziq, stop your treatment with Palynziq for a period of time, or prescribe medicine for you to take before your Palynziq injection to help reduce the symptoms of an allergic reaction.
The most common side effects of Palynziq include:
- injection site reactions, such as redness, itching, pain, bruising, rash, swelling, tenderness
- joint pain
- headache
- skin reactions that spread on your skin and last at least 14 days, such as itching, rash, redness
- nausea
- stomach pain
- vomiting
- cough
- mouth and throat pain
- itching
- diarrhea
- stuffy nose
- feel very tired
- dizziness
- anxiety
- low levels of phenylalanine in your blood
These are not all the possible side effects of Palynziq. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store Palynziq?
- Store Palynziq in the refrigerator at 36°F to 46°F (2°C to 8°C).
- If needed, you may store Palynziq at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days.
- Write the date that you remove Palynziq from the refrigerator on the carton.
- If stored at room temperature, do not put Palynziq back in the refrigerator.
- Keep Palynziq in the original carton to protect from light.
- Do not freeze or shake Palynziq.
- Throw away Palynziq if it has been kept at room temperature for 30 days and has not been used, or after the expiration date on the carton, whichever comes first.
Keep Palynziq and all medicines out of the reach of children.
General Information about the safe and effective use of Palynziq.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Palynziq for a condition for which it was not prescribed. Do not give Palynziq to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Palynziq that is written for health professionals.
What are the ingredients in Palynziq? Active ingredients: pegvaliase-pqpz
Inactive ingredients: sodium chloride, trans-cinnamic acid, tromethamine, and tromethamine hydrochlorideManufactured by: BioMarin Pharmaceutical Inc. Novato, CA 94949 US License No. 1649
For more information, go to http://www.Palynziq.com/ or call 1-866-906-6100.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 10/2020 -
INSTRUCTION FOR USEInstructions for Use - Palynziq (Pal-lin-zeek) (pegvaliase-pqpz) Injection for Subcutaneous Use - Single-Dose Prefilled Syringe - Read this Instructions for Use before you start using the ...
Instructions for Use
Palynziq (Pal-lin-zeek)
(pegvaliase-pqpz)
Injection for Subcutaneous Use
Single-Dose Prefilled Syringe
Read this Instructions for Use before you start using the Palynziq prefilled syringe and each time you get a new prescription. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Follow these instructions carefully while you are using Palynziq. If your healthcare provider decides that you (or your caregiver) can give the injections of Palynziq at home, your healthcare provider will show you (or your caregiver) how to inject Palynziq the right way. Your healthcare provider should watch you (or your caregiver) give the first Palynziq injection and monitor you for signs and symptoms of a severe allergic reaction (anaphylaxis). Do not inject Palynziq until your healthcare provider shows you (or your caregiver) how to inject Palynziq the right way and has watched you (or your caregiver) give your injection.
Talk to your healthcare provider if you (or your caregiver) have any questions about how to inject Palynziq the right way.
Do not share your prefilled syringes with anyone else. You may give an infection to them or get an infection from them.
Store your Palynziq prefilled syringe(s) in its original carton in the refrigerator. See “Storage of Palynziq” at the end of this Instructions for Use.
Supplies you will need for each injection of Palynziq (See Figure A):
-
Palynziq prefilled syringe(s) in sealed tray(s). Each tray contains 1 prefilled syringe. You may need more than 1 prefilled syringe for your prescribed dose.
-
1 gauze pad or cotton ball
-
1 alcohol pad
-
1 bandage
-
1 puncture resistant or sharps disposal container. See “Dispose of the used prefilled syringes” at the end of this Instructions for Use.
Figure A

Important things to know about using your Palynziq prefilled syringe:
-
Inject only 1 time with each Palynziq prefilled syringe. Do not use a Palynziq syringe more than 1 time.
-
Do not pull back on the plunger at any time.
-
Do not remove the needle cap until you are ready to inject.
Figure B below shows what the prefilled syringe looks like before use.
Figure B

Select the correct Palynziq prefilled syringe(s) for your dose. You may need more than 1 prefilled syringe for your prescribed dose. Your healthcare provider will tell you which syringe(s) to use. Ask your healthcare provider if you have any questions.
When you receive your Palynziq prefilled syringe(s), check that the name “Palynziq" appears on the carton(s).
-
Palynziq prefilled syringes come in 3 different strengths (See Figure C).
-
Before you inject Palynziq, check each carton and syringe to make sure you have the right prefilled syringe for your prescribed dose.
Figure C

Set up your injection
Step 1: Gather your supplies for the injection(s) (See Figure A) and place them on a clean flat surface.
Take out the number of cartons needed for your prescribed dose from the refrigerator.
Check the expiration date (EXP) on the carton(s) (See Figure D).
- If the expiration date has passed, do not use the prefilled syringe(s) in that carton. Call BioMarin at 1‑866‑906‑6100 or your healthcare provider for help with Palynziq.
Figure D

Step 2: Open the carton(s) and take out the sealed tray(s) that you need for your prescribed dose (See Figure E). You may need more than 1 prefilled syringe for your prescribed dose.
Place the sealed tray(s) on a clean, flat surface out of reach of children and pets.
Put the carton with any remaining trays back in the refrigerator.
Figure E

Step 3: Let the sealed tray(s) sit at room temperature for at least 30 minutes. Injecting Palynziq when cold can make the injection feel uncomfortable.
- Do not warm up the prefilled syringe in any other way other than letting it sit at room temperature. Do not warm in a microwave and do not place in hot water.
Let the sealed tray(s) sit at room temperature for at least 30 minutes before opening. Step 4: Peel off the cover from the tray (See Figure F).
Figure F

Step 5: Hold the middle of the prefilled syringe body and remove the prefilled syringe from the tray (See Figure G).
- Throw away the prefilled syringe if it looks damaged or used, and use a new prefilled syringe for your injection. See “Dispose of the used prefilled syringes” at the end of this Instructions for Use. Call BioMarin at 1‑866‑906‑6100 or your healthcare provider for help with Palynziq.
- Do not remove the needle cap from the prefilled syringe until Step 12.
- Do not shake or roll the syringe in your hands.
Figure G

Step 6: Check the prefilled syringe label to make sure you have the correct strength for your prescribed dose.
Check the prefilled syringe label to make sure you have the correct strength. Step 7: Look at the liquid through the viewing window (See Figure H).
- It is normal to see air bubbles. Do not flick or try to push the bubbles out.
The liquid should look clear and colorless to pale yellow. Throw away the prefilled syringe if the liquid is cloudy, discolored, or has particles in it and use a new prefilled syringe for your injection. See “Dispose of the used prefilled syringes” at the end of this Instructions for Use. Call BioMarin at 1‑866‑906‑6100 or your healthcare provider for help with Palynziq.
Figure H

Choose and prepare injection site
Step 8: Choose your injection site.
The recommended injection sites are:
- Front middle of the thighs.
- The abdomen except for the 2 inch (5 centimeter) area directly around the belly button (navel).
Figure I
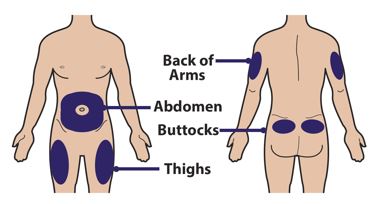
If a caregiver is giving the injection, the top of the buttocks and the back of the upper arms may also be used (See Figure I).
- Do not inject into moles, scars, birthmarks, bruises, rashes, or areas where the skin is hard, tender, red, damaged, burned, inflamed, or tattooed.
- If you need more than 1 injection for your dose, the injection sites should be at least 2 inches away from each other. The second injection site can be on the same part of the body or a different part of the body (See Figures I and J).
- For each injection, change (rotate) your injection sites. Choose an injection site that is at least 2 inches away from the injection site that you used for the last injection. It can be on the same part of the body or a different part of the body (See Figures I and J).
Figure J
Inject at least 2 inches apart

Step 9: Wash your hands well with soap and water before injecting Palynziq (See Figure K).
Figure K

Step 10: Clean the chosen site with an alcohol pad. Let the skin air dry for at least 10 seconds before injecting Palynziq (See Figure L).
- Do not touch the cleaned injection site.
- Do not remove the needle cap until you are ready to inject Palynziq.
Figure L
Inject Palynziq
Step 11: Hold the body of the prefilled syringe with 1 hand with the needle facing away from you (See Figure M).
- Do not use the prefilled syringe if it is dropped. Throw away the prefilled syringe if it is dropped and use a new prefilled syringe for your injection. See “Dispose of the used prefilled syringes” at the end of this Instructions for Use. Call BioMarin at 1‑866‑906‑6100 or your healthcare provider for help with Palynziq.
Figure M

Step 12: Pull the needle cap straight off the needle (See Figure N).
- Do not twist the needle cap during removal.
- Do not hold the prefilled syringe by the plunger or plunger head while taking the needle cap off.
- Before injecting Palynziq, check to make sure that the needle is not damaged or bent. Throw away the prefilled syringe if the needle is damaged or bent and use a new prefilled syringe for your injection. See “Dispose of the used prefilled syringes” at the end of this Instructions for Use. Call BioMarin at 1‑866‑906‑6100 or your healthcare provider for help with Palynziq.
You may see a drop of liquid on the tip of the needle. This is normal. Do not wipe it away. Throw the needle cap away in a puncture resistant or sharps disposal container.
Figure N

Step 13: Hold the body of the prefilled syringe in 1 hand between your thumb and index finger. Use your other hand to pinch up the skin around the injection site. Hold the skin firmly (See Figure O).
- Do not touch the plunger head while inserting the needle into the skin.
Figure O

Step 14: Use a quick motion to fully insert the needle into the pinched skin at a 45 to 90 degree angle (See Figure P).
Release the pinch of skin. Use that hand to hold the bottom of the prefilled syringe steady. Place the thumb of your other hand on the plunger head and place your index and middle fingers under the finger grips (See Figure P).
Figure P
Step 15: Use your thumb to push in the plunger slowly and steadily as far as it will go to inject all the medicine (See Figure Q). More pressure on the plunger may be needed to inject all the medicine for the 10 mg and 20 mg strength prefilled syringes.
Figure Q

Step 16: Slowly move your thumb up to release the plunger. The needle will automatically be covered by the prefilled syringe body (See Figure R).
Figure R

Treat injection site
Step 17: Treat injection site (if needed).
If you see drops of blood at the injection site, press a cotton ball or gauze over the injection site and hold for about 10 seconds. You may cover the injection site with a small adhesive bandage if needed.
If more than 1 prefilled syringe is needed for your prescribed dose:
Step 18: If your healthcare provider tells you to use more than 1 prefilled syringe for your prescribed dose, repeat Steps 4 to 17 listed above for each prefilled syringe that you use.
- Note: Do not give the injection in the same spot. The injection sites should be at least 2 inches away from each other. See Step 8 for information on choosing an injection site.
If you need to use more than 1 prefilled syringe for your prescribed dose, repeat Steps 4 to 17 right away for each prefilled syringe you use. Dispose of the used prefilled syringes
Step 19: Put your used prefilled syringes in a puncture resistant container, such as an FDA‑cleared sharps disposal container immediately after use (See Figure S).
Do not throw away (dispose of) prefilled syringes in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Note: Keep sharps disposal container out of the reach of children and pets.
Figure S

Storage of Palynziq
Figure T

- Store Palynziq in the refrigerator at 36°F to 46°F (2°C to 8°C) (See Figure T).
- If needed, you may store Palynziq at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days.
- Write the date that you remove Palynziq from the refrigerator on the carton.
- If stored at room temperature, do not put Palynziq back in the refrigerator.
- Keep Palynziq in the original carton to protect from light.
- Do not freeze or shake Palynziq.
- Throw away Palynziq if it has been kept at room temperature for 30 days and has not been used, or after the expiration date on the carton, whichever comes first.
Keep Palynziq and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
BioMarin Pharmaceutical Inc.
Novato, CA 94949
U.S. License No. 1649
Revised: 10/2020
Close -
-
PALYNZIQ - 2.5 MG/0.5 MLNDC 68135-058-90 Rx only - PalynziqTM - (pegvaliase-pqpz) Injection - 2.5 mg/0.5 mL - For Subcutaneous Use Only - Contains one x 2.5 mg/0.5 mL single-dose prefilled ...
NDC 68135-058-90 Rx only
PalynziqTM
(pegvaliase-pqpz)
Injection
2.5 mg/0.5 mL
For Subcutaneous Use Only
Contains one x 2.5 mg/0.5 mL single-dose prefilled syringe.
Discard after use.
ATTENTION: Dispense the enclosed Medication Guide to each patient.



Close -
PALYNZIQ - 10 MG/0.5MLNDC 68135-756-20 Rx only - PalynziqTM - (pegvaliase-pqpz) Injection - 10 mg/0.5 mL - For Subcutaneous Use Only - Contains one x 10 mg/0.5 mL single-dose prefilled ...
NDC 68135-756-20 Rx only
PalynziqTM
(pegvaliase-pqpz)
Injection
10 mg/0.5 mL
For Subcutaneous Use Only
Contains one x 10 mg/0.5 mL single-dose prefilled syringe.
Discard after use.
ATTENTION: Dispense the enclosed Medication Guide to each patient.
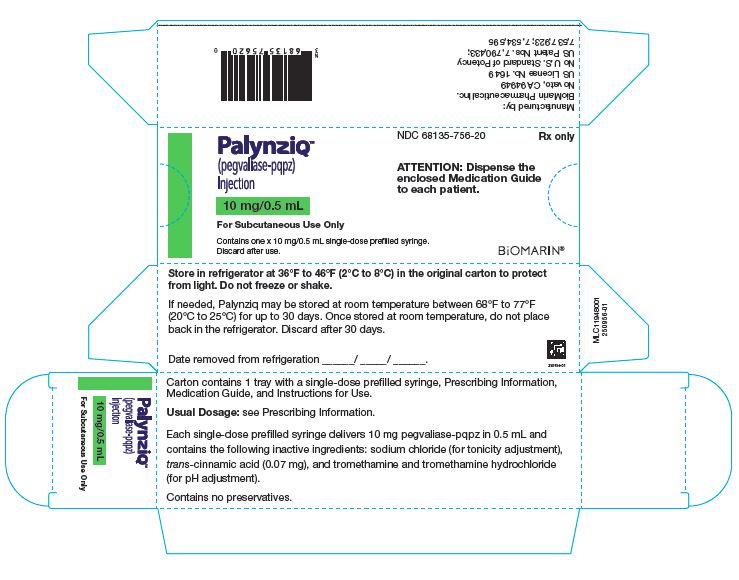
 Close
Close
-
PALYNZIQ (SINGLE PACK) - 20 MG/MLNDC 68135-673-40 Rx only - PalynziqTM - (pegvaliase-pqpz) Injection - 20 mg/mL - For Subcutaneous Use Only - Contains one x 20 mg/mL single-dose prefilled syringe. Discard ...
NDC 68135-673-40 Rx only
PalynziqTM
(pegvaliase-pqpz)
Injection
20 mg/mL
For Subcutaneous Use Only
Contains one x 20 mg/mL single-dose prefilled syringe.
Discard after use.
ATTENTION: Dispense the enclosed Medication Guide to each patient.
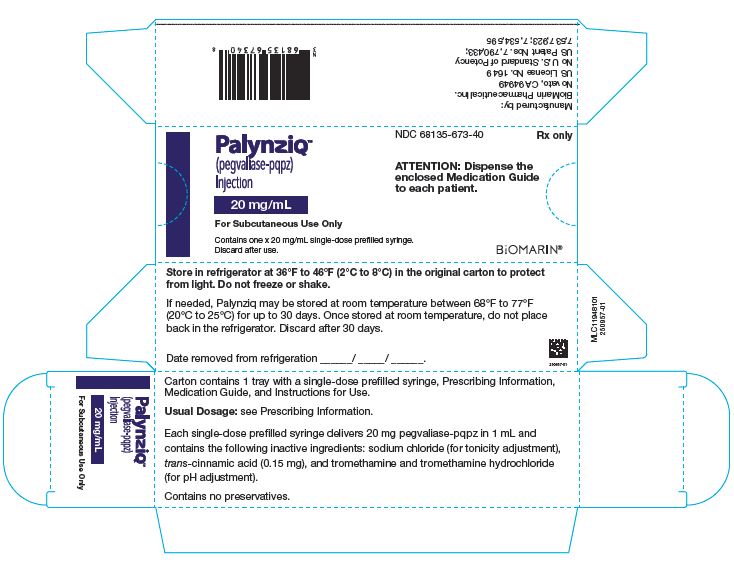

Close

-
PALYNZIQ (MULTI-PACK) - 20 MG/MLNDC 68135-673-45 Rx only - PalynziqTM - (pegvaliase-pqpz) Injection - 20 mg/mL - For Subcutaneous Use Only - Contains ten 20 mg/mL single-dose prefilled syringes. Discard ...
NDC 68135-673-45 Rx only
PalynziqTM
(pegvaliase-pqpz)
Injection
20 mg/mL
For Subcutaneous Use Only
Contains ten 20 mg/mL single-dose prefilled syringes.
Discard after use.
ATTENTION: Dispense the enclosed Medication Guide to each patient.
Close
-
INGREDIENTS AND APPEARANCEProduct Information
PALYNZIQ pegvaliase-pqpz injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68135-058 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEGVALIASE (UNII: N6UAH27EUV) (PEGVALIASE - UNII:N6UAH27EUV) PEGVALIASE 2.5 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-058-90 1 in 1 CARTON 05/24/2018 1 NDC:68135-058-89 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761079 05/24/2018 PALYNZIQ pegvaliase-pqpz injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68135-756 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEGVALIASE (UNII: N6UAH27EUV) (PEGVALIASE - UNII:N6UAH27EUV) PEGVALIASE 10 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-756-20 1 in 1 CARTON 05/24/2018 1 NDC:68135-756-19 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761079 05/24/2018 PALYNZIQ pegvaliase-pqpz injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68135-673 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PEGVALIASE (UNII: N6UAH27EUV) (PEGVALIASE - UNII:N6UAH27EUV) PEGVALIASE 20 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-673-40 1 in 1 CARTON 05/24/2018 1 NDC:68135-673-39 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:68135-673-45 10 in 1 CARTON 05/24/2018 2 NDC:68135-673-39 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761079 05/24/2018
CloseLabeler - BioMarin Pharmaceutical Inc. (079722386)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
PALYNZIQ- pegvaliase-pqpz injection, solution
Number of versions: 7
RxNorm
PALYNZIQ- pegvaliase-pqpz injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2046365 | pegvaliase-pqpz 10 MG in 0.5 mL Prefilled Syringe | PSN |
| 2 | 2046365 | 0.5 ML pegvaliase-pqpz 20 MG/ML Prefilled Syringe | SCD |
| 3 | 2046365 | pegvaliase-pqpz 10 MG per 0.5 ML Prefilled Syringe | SY |
| 4 | 2046370 | Palynziq 10 MG in 0.5 mL Prefilled Syringe | PSN |
| 5 | 2046370 | 0.5 ML pegvaliase-pqpz 20 MG/ML Prefilled Syringe [Palynziq] | SBD |
| 6 | 2046370 | 0.5 ML Palynziq 20 MG/ML Prefilled Syringe | SY |
| 7 | 2046370 | Palynziq 10 MG per 0.5 ML Prefilled Syringe | SY |
| 8 | 2046374 | pegvaliase-pqpz 2.5 MG in 0.5 mL Prefilled Syringe | PSN |
| 9 | 2046374 | 0.5 ML pegvaliase-pqpz 5 MG/ML Prefilled Syringe | SCD |
| 10 | 2046374 | pegvaliase-pqpz 2.5 MG per 0.5 ML Prefilled Syringe | SY |
| 11 | 2046376 | Palynziq 2.5 MG in 0.5 mL Prefilled Syringe | PSN |
| 12 | 2046376 | 0.5 ML pegvaliase-pqpz 5 MG/ML Prefilled Syringe [Palynziq] | SBD |
| 13 | 2046376 | 0.5 ML Palynziq 5 MG/ML Prefilled Syringe | SY |
| 14 | 2046376 | Palynziq 2.5 MG per 0.5 ML Prefilled Syringe | SY |
| 15 | 2046379 | pegvaliase-pqpz 20 MG in 1 mL Prefilled Syringe | PSN |
| 16 | 2046379 | 1 ML pegvaliase-pqpz 20 MG/ML Prefilled Syringe | SCD |
| 17 | 2046379 | pegvaliase-pqpz 20 MG per 1 ML Prefilled Syringe | SY |
| 18 | 2046380 | Palynziq 20 MG in 1 mL Prefilled Syringe | PSN |
| 19 | 2046380 | 1 ML pegvaliase-pqpz 20 MG/ML Prefilled Syringe [Palynziq] | SBD |
| 20 | 2046380 | 1 ML Palynziq 20 MG/ML Prefilled Syringe | SY |
| 21 | 2046380 | Palynziq 20 MG per 1 ML Prefilled Syringe | SY |
Get Label RSS Feed for this Drug
PALYNZIQ- pegvaliase-pqpz injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=6dba844a-db02-44f8-8593-ce497ed9406c
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
PALYNZIQ- pegvaliase-pqpz injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 68135-058-89 |
| 2 | 68135-058-90 |
| 3 | 68135-673-39 |
| 4 | 68135-673-40 |
| 5 | 68135-673-45 |
| 6 | 68135-756-19 |
| 7 | 68135-756-20 |










