Label: OZURDEX- dexamethasone implant
- NDC Code(s): 0023-3348-07, 0023-3348-08
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OZURDEX® safely and effectively. See full prescribing information for OZURDEX®. OZURDEX® (dexamethasone intravitreal ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
1.1 - Retinal Vein Occlusion - OZURDEX® (dexamethasone intravitreal implant) is indicated for the treatment of macular edema following branch retinal vein occlusion (BRVO) or central ...
-
2
DOSAGE AND ADMINISTRATION
2.1 - General Dosing Information - For ophthalmic intravitreal injection. 2.2 - Administration - The intravitreal injection procedure should be carried out under controlled ...
-
3
DOSAGE FORMS AND STRENGTHS
Intravitreal implant containing dexamethasone 0.7 mg in the NOVADUR® solid polymer drug delivery system.
-
4
CONTRAINDICATIONS
4.1 - Ocular or Periocular Infections - OZURDEX® (dexamethasone intravitreal implant) is contraindicated in patients with active or suspected ocular or periocular infections including ...
-
5
WARNINGS AND PRECAUTIONS
5.1 - Intravitreal Injection-related Effects - Intravitreal injections, including those with OZURDEX®, have been associated with endophthalmitis, eye inflammation, increased intraocular ...
-
6
ADVERSE REACTIONS
6.1 - Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - There are no adequate and well-controlled studies with OZURDEX® in pregnant women. Topical ocular administration of dexamethasone in mice and rabbits ...
-
11
DESCRIPTION
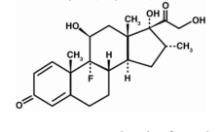
OZURDEX® is a sterile intravitreal implant containing 0.7 mg (700 mcg) dexamethasone in the NOVADUR® solid polymer sustained-release drug delivery system which does not contain an antimicrobial ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Dexamethasone, a corticosteroid, has been shown to suppress inflammation by inhibiting multiple inflammatory cytokines resulting in decreased edema, fibrin ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been conducted to determine whether OZURDEX® (dexamethasone intravitreal implant) has the potential ...
-
14
CLINICAL STUDIES
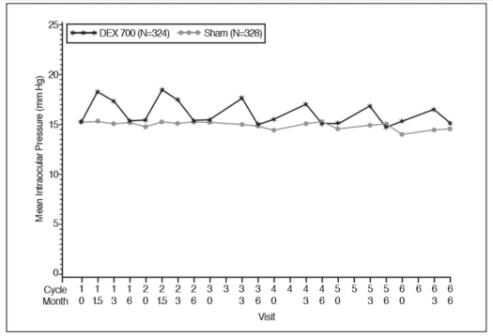
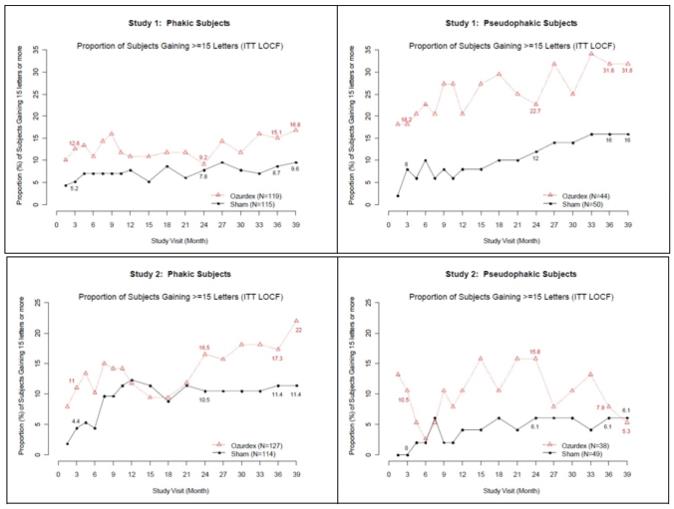
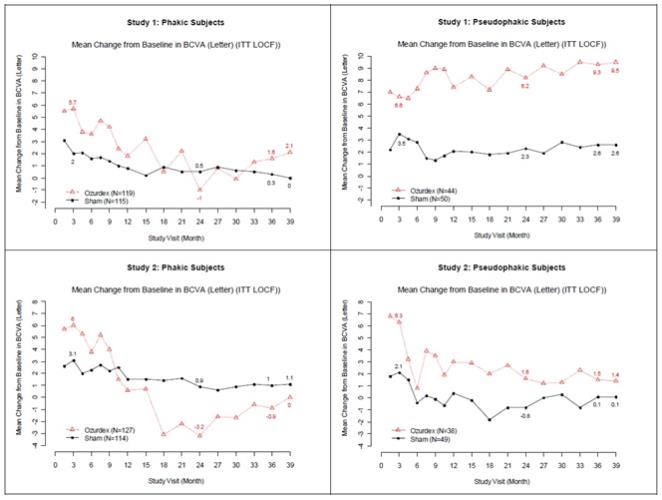
Retinal Vein Occlusion - The efficacy of OZURDEX® for the treatment of macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO) was assessed in two ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
OZURDEX® (dexamethasone intravitreal implant) 0.7 mg is supplied in a foil pouch with 1 single-use plastic applicator, NDC 0023-3348-07. Storage: Store at 15oC to 30oC (59oF to 86oF).
-
17
PATIENT COUNSELING INFORMATION
Steroid-related Effects - Advise patients that a cataract may occur after repeated treatment with OZURDEX®. If this occurs, advise patients that their vision will decrease, and they will need an ...
-
PRINCIPAL DISPLAY PANEL
NDC 0023-3348-07 - Ozurdex® (dexamethasone - intravitreal implant) 0.7 mg - Allergan - For Intravitreal Injection - Contents include: One Sterile, Use Applicator - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information