Label: TECHNESCAN HDP- technetium tc 99m oxidronate injection, powder, lyophilized, for solution
- NDC Code(s): 69945-091-20, 69945-091-40
- Packager: Curium US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONTechnescan™ HDP is supplied as a lyophilized powder, packaged under nitrogen in vials for intravenous administration after reconstitution with ADDITIVE-FREE sodium pertechnetate Tc 99m. Each vial ...
-
PHYSICAL CHARACTERISTICSTechnetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.1The principal photon that is useful for detection and imaging is listed in Table 1. Table 1. Principal ...
-
EXTERNAL RADIATIONThe specific gamma ray constant for Technetium Tc 99m is 0.78 R/mCi-hr at 1 cm. The first half-value thickness of lead (Pb) for Technetium Tc 99m is 0.017 cm. A range of values for the relative ...
-
CLINICAL PHARMACOLOGYDuring the 24 hours following injection, Technetium Tc 99m-labeled Technescan HDP is rapidly cleared from blood and other non-osseous tissues and accumulates in the skeleton and urine in humans ...
-
INDICATIONS AND USAGETechnescan HDP Tc 99m is a diagnostic skeletal imaging agent used to demonstrate areas of altered osteogenesis in adult and pediatric patients.
-
CONTRAINDICATIONSNone known.
-
WARNINGSTechnetium Tc 99m Oxidronate may cause life-threatening hypersensitivity reactions. Have cardiopulmonary resuscitation equipment and personnel available and monitor all patients for ...
-
PRECAUTIONSGeneral - The components of the kit are sterile and non-pyrogenic. It is essential that the user follow the directions carefully and adhere to strict aseptic procedures during preparation. Sodium ...
-
ADVERSE REACTIONSHypersensitivity reactions, including life-threatening reactions, as well as nausea, vomiting and injection site reactions, have been infrequently reported with Technetium Tc 99m Oxidronate ...
-
DOSAGE AND ADMINISTRATIONGeneral Instructions - The recommended adult dose of Technetium Tc 99m-labeled Technescan HDP is 555 MBq (15 mCi) with a range of 370 to 740 MBq (10 to 20 mCi). The recommended pediatric dose is ...
-
RADIATION DOSIMETRYThe estimated absorbed radiation doses from an intravenous injection of Technetium Tc 99m-labeled Technescan HDP are shown in Table 4. Table 4. Estimated Absorbed Radiation ...
-
PREPARATIONS FOR USEAll procedures should be conducted using waterproof gloves. Use shielded syringe during transport and administration of Tc 99m solutions. Remove plastic disc from Technescan HDP vial and cleanse ...
-
UNIT DOSE PREPARATIONPreparing a dose for a single adult patient or for a pediatric patient - To minimize volume injected and to ensure optimum solution concentration, reconstitute the vial contents in 3 to 6 mL of ...
-
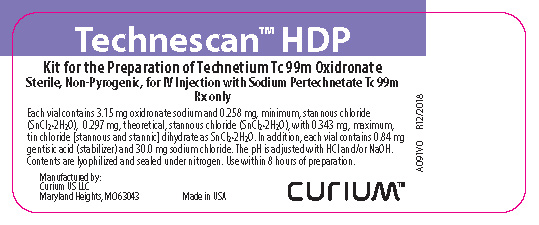
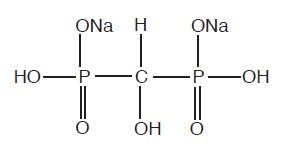
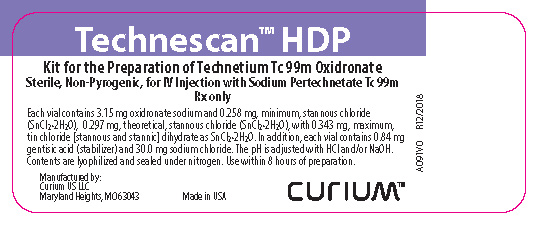
HOW SUPPLIEDTechnescan HDP is supplied as a lyophilized powder packaged in vials. Each vial contains 3.15 mg oxidronate sodium and 0.258 mg, minimum, stannous chloride (SnCl2•2H2O), 0.297 mg, theoretical ...
-
PRINCIPAL DISPLAY PANELTechnescan™ HDP Kit for the Preparation of Technetium Tc 99m Oxidronate - Sterile, Non-Pyrogenic, for IV Injection with Sodium Pertechnetate Tc 99m - Rx only - Each vial contains 3.15 mg oxidronate ...
-
INGREDIENTS AND APPEARANCEProduct Information