Label: OXBRYTA- voxelotor tablet, film coated
OXBRYTA- voxelotor tablet, for suspension
- NDC Code(s): 72786-101-01, 72786-102-02, 72786-102-03, 72786-111-02, view more
- Packager: Global Blood Therapeutics, Inc, A subsidiary of Pfizer Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OXBRYTA safely and effectively. See full prescribing information for OXBRYTA. OXBRYTA® (voxelotor) tablets, for oral use - OXBRYTA ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEOXBRYTA is indicated for the treatment of sickle cell disease (SCD) in adults and pediatric patients 4 years of age and older. This indication is approved under accelerated approval based on ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Adults and Pediatric Patients 12 Years and Older - The recommended dosage of OXBRYTA is 1,500 mg orally once daily. 2.2 Recommended Dosage for Pediatric Patients 4 ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 300 mg light purple to purple, oval shaped, biconvex, debossed with "G 300" on one side. Tablets: 500 mg light yellow to yellow, oval shaped, biconvex, debossed with "GBT 500" on one ...
-
4 CONTRAINDICATIONSOXBRYTA is contraindicated in patients with a history of serious drug hypersensitivity reaction to voxelotor or excipients. Clinical manifestations may include generalized rash, urticaria, mild ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Serious hypersensitivity reactions after administration of OXBRYTA have occurred in <1% of patients treated. Clinical manifestations may include generalized rash ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reaction is discussed in other sections of the labeling: • Hypersensitivity Reactions [see Contraindications (4) and Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on Voxelotor - Strong or Moderate CYP3A4 Inducers - Coadministration of strong or moderate CYP3A4 inducers may decrease voxelotor plasma and whole blood ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on OXBRYTA use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or ...
-
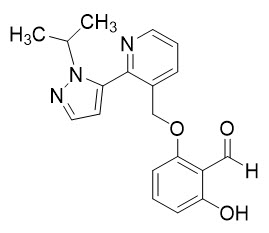
11 DESCRIPTIONOXBRYTA contains voxelotor, a hemoglobin S polymerization inhibitor. The chemical name of voxelotor is 2-hydroxy-6-((2-(1-isopropyl-1H-pyrazol-5-yl)pyridin-3-yl)methoxy)benzaldehyde with a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Voxelotor is a hemoglobin S (HbS) polymerization inhibitor that binds to HbS with a 1:1 stoichiometry and exhibits preferential partitioning to red blood cells (RBCs) ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Voxelotor was not carcinogenic in a 26-week study in RasH2 transgenic mice at oral doses of 30, 150, or 500 mg/kg/day. Voxelotor was ...
-
14 CLINICAL STUDIES14.1 Adults and Pediatric Patients 12 Years and Older - The efficacy and safety of OXBRYTA in SCD was evaluated in HOPE, a Phase 3 randomized, double-blind, placebo-controlled, multicenter trial ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGThe 300 mg tablet is film-coated, light purple to purple, oval shaped, biconvex, debossed with "G 300" on one side, available in: • Bottles of 60 tablets with one desiccant canister, a polyester ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Hypersensitivity Reactions - Advise patients that serious hypersensitivity reactions ...
-
SPL UNCLASSIFIED SECTIONThis product's label may have been updated. For most recent prescribing information, please visit www.pfizer.com. For medical information about OXBRYTA, please visit www.pfizermedinfo.com or call ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration - Revised: 08/2023 - PATIENT INFORMATION - OXBRYTA® (ox brye ta) (voxelotor ...
-
INSTRUCTIONS FOR USEThis Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 08/2023 - INSTRUCTIONS FOR USE - OXBRYTA® [ox brye ta] (voxelotor) tablets for oral ...
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle LabelNDC 72786-101-01 - Pfizer - Oxbryta® (voxelotor) tablets - 500 mg - Swallow tablets whole. Do not cut, crush, or chew the tablets. 90 tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle LabelNDC 72786-111-03 - Pfizer - Oxbryta® (voxelotor) tablets for oral - suspension - 300 mg - 90 tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle Label - 72786-102NDC 72786-102-03 - Rx only - Oxbryta® (voxelotor) tablets - 300 mg - Swallow tablets whole. Do not cut, crush, or chew the tablets. 90 tablets
-
INGREDIENTS AND APPEARANCEProduct Information