Label: ISTURISA- osilodrostat tablet, coated
ISTURISA- osilodrostat tablet, coated
ISTURISA- osilodrostat tablet, coated
- NDC Code(s): 55292-320-20, 55292-320-60, 55292-321-20, 55292-321-60, view more
- Packager: Recordati Rare Diseases, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 25, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ISTURISA® safely and effectively. See full prescribing information for ISTURISA. ISTURISA (osilodrostat) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEISTURISA is indicated for the treatment of endogenous hypercortisolemia in adults with Cushing's syndrome for whom surgery is not an option or has not been curative.
-
2 DOSAGE AND ADMINISTRATION2.1 Laboratory Testing Prior to ISTURISA Initiation - Correct hypokalemia and hypomagnesemia prior to starting ISTURISA [see Warnings and Precautions (5.2, 5.3)]. Obtain baseline ...
-
3 DOSAGE FORMS AND STRENGTHSISTURISA is available as: 1 mg tablets: Pale yellow, unscored, round, biconvex with beveled edge tablet, debossed "1" on one side. 5 mg tablets: Yellow, unscored, round, biconvex with beveled ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypocortisolism - ISTURISA lowers cortisol levels and can lead to hypocortisolism and sometimes life-threatening adrenal insufficiency. Lowering of cortisol can cause nausea, vomiting ...
-
6 ADVERSE REACTIONSClinically significant adverse reactions that appear in other sections of the labeling include: Hypocortisolism [see Warnings and Precautions (5.1)] QT Prolongation [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on ISTURISA - The effect of other drugs on ISTURISA can be found in Table 2. Table 2: Effect of Other Drugs on ISTURISA - CYP3A4 Inhibitors - Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on osilodrostat use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse ...
-
10 OVERDOSAGEOverdosage may result in severe hypocortisolism. Signs and symptoms suggestive of hypocortisolism may include nausea, vomiting, fatigue, low blood pressure, abdominal pain, loss of appetite ...
-
11 DESCRIPTIONISTURISA (osilodrostat) is a cortisol synthesis inhibitor. The chemical name of osilodrostat is 4-[(5R)-6,7-Dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl]-3-fluorobenzonitrile dihydrogen ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Osilodrostat is a cortisol synthesis inhibitor. It inhibits 11beta-hydroxylase (CYP11B1), the enzyme responsible for the final step of cortisol biosynthesis in the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - Carcinogenesis - Carcinogenicity studies were conducted in Wistar Han rats and CD1 mice. Hepatocellular adenomas and carcinomas ...
-
14 CLINICAL STUDIESThe safety and efficacy of ISTURISA was assessed in a 48-week, multicenter study (called the Core Period) that consisted of four study periods as follows: Period 1: 12-week, open-label, dose ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ISTURISA (osilodrostat) tablets are supplied as follows: Tablet StrengthDescriptionPackage ConfigurationNDC No. 1 mgPale yellow, unscored, round, biconvex with ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information). Monitoring - Instruct patients on the importance of laboratory monitoring and adhering to their return visit ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Recordati Rare Diseases Inc. Bridgewater, NJ 08807 USA - © Recordati
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - ISTURISA® (is tur ee' sah) (osilodrostat) tablets - This Patient Information has been approved by the U.S. Food and Drug Administration.Revised ...
-
PRINCIPAL DISPLAY PANEL - 1 mg Blister Pack Carton - 330NDC 55292-330-60 - Isturisa® 1 mg - (osilodrostat) tablets - Rx only - Each tablet contains 1 mg osilodrostat (as osilodrostat phosphate). 60 film-coated tablets - RECORDATI - RARE DISEASES
-
PRINCIPAL DISPLAY PANEL - 5 mg Blister Pack Carton - 331NDC 55292-331-60 - Isturisa® 5 mg - (osilodrostat) tablets - Rx only - Each tablet contains 5 mg osilodrostat (as osilodrostat phosphate). 60 film-coated tablets - RECORDATI - RARE DISEASES
-
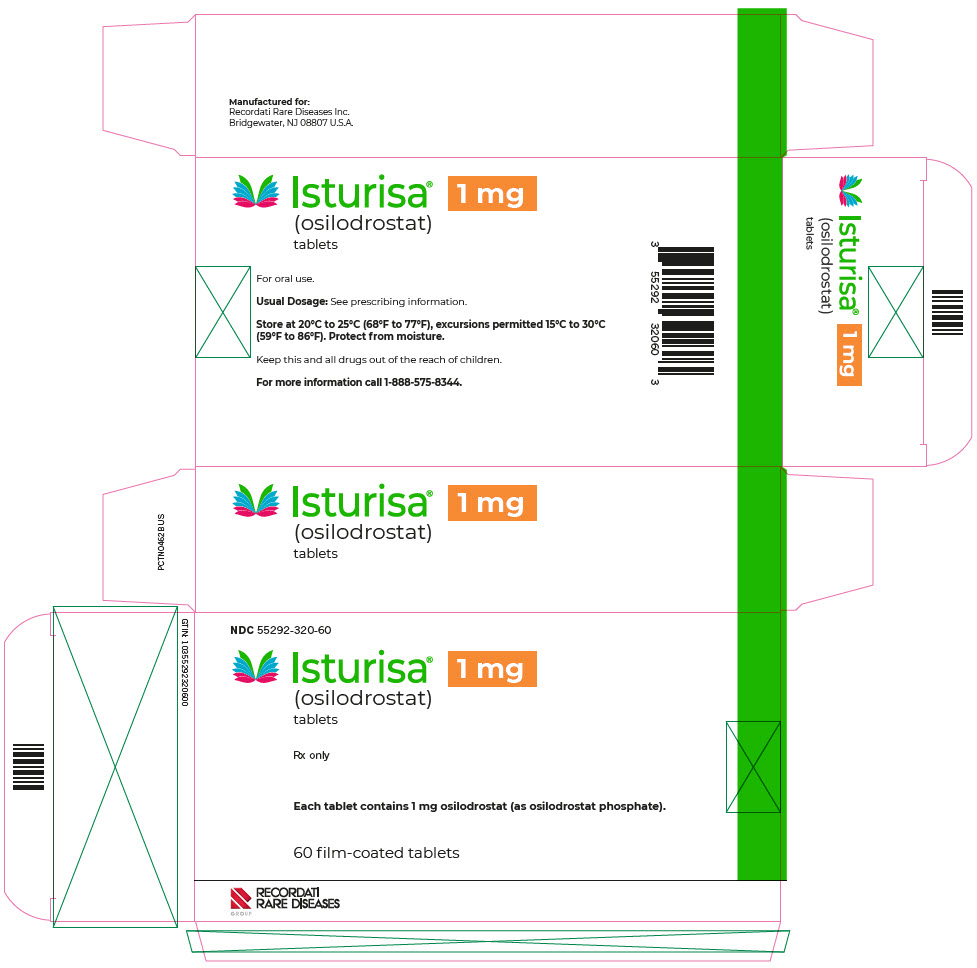
PRINCIPAL DISPLAY PANEL - 1 mg Blister Pack CartonNDC 55292-320-60 - Isturisa® (osilodrostat) tablets - 1 mg - Rx only - Each tablet contains 1 mg osilodrostat (as osilodrostat phosphate). 60 film-coated tablets - RECORDATI - RARE DISEASES
-
PRINCIPAL DISPLAY PANEL - 5 mg Blister Pack CartonNDC 55292-321-60 - Isturisa® (osilodrostat) tablets - 5 mg - Rx only - Each tablet contains 5 mg osilodrostat (as osilodrostat phosphate). 60 film-coated tablets - RECORDATI - RARE DISEASES
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information