Label: ORKAMBI- lumacaftor and ivacaftor tablet, film coated
ORKAMBI- lumacaftor and ivacaftor granule
-
NDC Code(s):
51167-122-01,
51167-500-02,
51167-700-02,
51167-809-01, view more51167-900-01
- Packager: Vertex Pharmaceuticals Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ORKAMBI safely and effectively. See full prescribing information for ORKAMBI. ORKAMBI® (lumacaftor and ivacaftor) tablets, for ...These highlights do not include all the information needed to use ORKAMBI safely and effectively. See full prescribing information for ORKAMBI.
ORKAMBI® (lumacaftor and ivacaftor) tablets, for oral use
ORKAMBI® (lumacaftor and ivacaftor) oral granules
Initial U.S. Approval: 2015INDICATIONS AND USAGE
ORKAMBI is a combination of ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator, and lumacaftor, indicated for the treatment of cystic fibrosis (CF) in patients aged 1 year and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene. (1)
Limitations of Use:
The efficacy and safety of ORKAMBI have not been established in patients with CF other than those homozygous for the F508del mutation. (1)
DOSAGE AND ADMINISTRATION
Age Group Weight Dose Administration 1 through 2 years 7 kg to < 9 kg 1 packet of lumacaftor 75 mg/ivacaftor 94 mg granules Mixed with one teaspoon (5 mL) of soft food or liquid and administered orally every 12 hours with fat-containing food 9 kg to < 14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg granules ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg granules 2 through 5 years <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg granules ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg granules 6 through 11 years - 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg
(lumacaftor 200 mg/ivacaftor 250 mg per dose)Taken orally every 12 hours with fat-containing food 12 years and older - 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg
(lumacaftor 400 mg/ivacaftor 250 mg per dose)DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Use in patients with advanced liver disease: ORKAMBI should be used with caution in these patients and only if the benefits are expected to outweigh the risks. If ORKAMBI is used in these patients, they should be closely monitored after the initiation of treatment and the dosage should be reduced. Liver function decompensation, including liver failure leading to death, has been reported in CF patients with pre-existing cirrhosis with portal hypertension. (2.2, 5.1, 6.1)
- Liver-related events: Elevated transaminases (ALT/AST) have been observed in some cases associated with elevated bilirubin. Measure serum transaminases and bilirubin before initiating ORKAMBI, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of ALT, AST, or bilirubin elevations, more frequent monitoring should be considered. Interrupt dosing in patients with ALT or AST >5 × upper limit of normal (ULN), or ALT or AST >3 × ULN with bilirubin >2 × ULN. Following resolution, consider the benefits and risks of resuming dosing. (5.2, 6.1)
- Hypersensitivity reactions: Angioedema and anaphylaxis have been reported with ORKAMBI in the postmarketing setting. Initiate appropriate therapy in the event of a hypersensitivity reaction. (5.3)
- Respiratory events: Chest discomfort, dyspnea, and respiration abnormal were observed more commonly during initiation of ORKAMBI. Clinical experience in patients with percent predicted FEV1 (ppFEV1) <40 is limited, and additional monitoring of these patients is recommended during initiation of therapy. (5.4, 6.1)
- Blood pressure: Increased blood pressure has been observed in some patients. Periodically monitor blood pressure in all patients. (5.5, 6.1)
- Drug interactions: Use with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index may decrease systemic exposure of the medicinal products and co-administration is not recommended. Hormonal contraceptives should not be relied upon as an effective method of contraception and their use is associated with increased menstruation-related adverse reactions. Use with strong CYP3A inducers may diminish exposure of ivacaftor, which may diminish its effectiveness; therefore, co-administration is not recommended. (5.6, 6.1, 7, 12.3)
- Cataracts: Non-congenital lens opacities/cataracts have been reported in pediatric patients treated with ORKAMBI and ivacaftor, a component of ORKAMBI. Baseline and follow-up examinations are recommended in pediatric patients initiating ORKAMBI. (5.7)
ADVERSE REACTIONS
The most common adverse reactions to ORKAMBI (occurring in ≥5% of patients with CF homozygous for the F508del mutation in the CFTR gene) were dyspnea, nasopharyngitis, nausea, diarrhea, upper respiratory tract infection, fatigue, respiration abnormal, blood creatine phosphokinase increased, rash, flatulence, rhinorrhea, influenza. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Vertex Pharmaceuticals Incorporated at 1-877-634-8789 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults and Pediatric Patients Aged 1 Year and Older
2.2 Dosage Adjustment for Patients with Hepatic Impairment
2.3 Dosage Adjustment for Patients Taking CYP3A Inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Use in Patients with Advanced Liver Disease
5.2 Liver-related Events
5.3 Hypersensitivity Reactions, Including Anaphylaxis
5.4 Respiratory Events
5.5 Effect on Blood Pressure
5.6 Drug Interactions
5.7 Cataracts
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Inhibitors of CYP3A
7.2 Inducers of CYP3A
7.3 CYP3A Substrates
7.4 CYP2B6 and CYP2C Substrates
7.5 Digoxin and Other P-gp Substrates
7.6 Anti-allergics and Systemic Corticosteroids
7.7 Antibiotics
7.8 Antifungals
7.9 Anti-inflammatories
7.10 Antidepressants
7.11 Hormonal Contraceptives
7.12 Oral Hypoglycemics
7.13 Proton Pump Inhibitors, H2 Blockers, Antacids
7.14 Warfarin
7.15 Concomitant Drugs That Do Not Need Dosage Adjustment
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 Patients with Severe Lung Dysfunction
8.9 Patients After Organ Transplantation
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGEORKAMBI is indicated for the treatment of cystic fibrosis (CF) in patients aged 1 year and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown ...
ORKAMBI is indicated for the treatment of cystic fibrosis (CF) in patients aged 1 year and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene.
CloseLimitations of Use
The efficacy and safety of ORKAMBI have not been established in patients with CF other than those homozygous for the F508del mutation.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage in Adults and Pediatric Patients Aged 1 Year and Older - The recommended dosage of ORKAMBI in adults and pediatric patients aged one year and older is based on patient's ...
2.1 Recommended Dosage in Adults and Pediatric Patients Aged 1 Year and Older
The recommended dosage of ORKAMBI in adults and pediatric patients aged one year and older is based on patient's age and weight as described in Table 1.
Table 1: Recommended Oral Dosage of ORKAMBI in Patients Aged 1 Year and Older Age Group Weight ORKAMBI Daily Dose (every 12 hours) Morning Dose Evening Dose 1 through 2 years 7 kg to <9 kg 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules 9 kg to <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 2 through 5 years <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 6 through 11 years - 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg
(lumacaftor 200 mg/ivacaftor 250 mg per dose)2 tablets of lumacaftor 100 mg/ivacaftor 125 mg
(lumacaftor 200 mg/ivacaftor 250 mg per dose)12 years and older - 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg
(lumacaftor 400 mg/ivacaftor 250 mg per dose)2 tablets of lumacaftor 200 mg/ivacaftor 125 mg
(lumacaftor 400 mg/ivacaftor 250 mg per dose)Administration Instructions for ORKAMBI Oral Granules
The entire content of each packet of oral granules should be mixed with one teaspoon (5 mL) of age-appropriate soft food or liquid and the mixture completely consumed. Some examples of soft foods or liquids include puréed fruits or vegetables, flavored yogurt or pudding, applesauce, water, milk, breast milk, infant formula or juice. Food should be at room temperature or below. Each packet is for single use only. Once mixed, the product has been shown to be stable for one hour, and therefore should be ingested during this period.
Administration with Fat-Containing Food for ORKAMBI Tablets and Oral Granules
A fat-containing meal or snack should be consumed just before or just after dosing for all formulations. Examples of appropriate fat-containing foods include eggs, avocados, nuts, butter, peanut butter, cheese pizza, breast milk, infant formula, whole-milk dairy products (such as whole milk, cheese, and yogurt), etc.
Missed Dose
If a patient misses a dose and remembers the missed dose within 6 hours, the patient should take the dose with fat-containing food. If more than 6 hours elapsed after the recommended dosing time, the patient should skip that dose and resume the normal schedule for the following dose. A double dose should not be taken to make up for the forgotten dose [see Clinical Pharmacology (12.3) and Patient Counseling Information (17)].
2.2 Dosage Adjustment for Patients with Hepatic Impairment
For dosage adjustment for patients with hepatic impairment, refer to Table 2.
Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a maximum dose of 1 tablet in the morning and 1 tablet in the evening or less frequently, or 1 packet of oral granules once daily or less frequently in patients with severe hepatic impairment after weighing the risks and benefits of treatment [see Dosage and Administration (2.1), Use in Specific Populations (8.6), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
Table 2: Recommended Dosage for Patients with Hepatic Impairment Age Group Weight Morning Dose Evening Dose - *
- or less frequently.
Mild (Child-Pugh Class A) 1 through 2 years 7 kg to <9 kg 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules 9 kg to <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 2 through 5 years <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 6 through 11 years - 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg
(lumacaftor 200 mg/ivacaftor 250 mg per dose)2 tablets of lumacaftor 100 mg/ivacaftor 125 mg
(lumacaftor 200 mg/ivacaftor 250 mg per dose)12 years and older - 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg
(lumacaftor 400 mg/ivacaftor 250 mg per dose)2 tablets of lumacaftor 200 mg/ivacaftor 125 mg
(lumacaftor 400 mg/ivacaftor 250 mg per dose)Moderate (Child-Pugh Class B) 1 through 2 years 7 kg to <9 kg 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules every other day 9 kg to <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules every other day ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules every other day 2 through 5 years <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules every other day ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules every other day 6 through 11 years - 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg
(lumacaftor 200 mg/ivacaftor 250 mg per dose)1 tablet of lumacaftor 100 mg/ivacaftor 125 mg 12 years and older - 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg
(lumacaftor 400 mg/ivacaftor 250 mg per dose)1 tablet of lumacaftor 200 mg/ivacaftor 125 mg Severe (Child-Pugh Class C) 1 through 2 years 7 kg to <9 kg 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules* N/A 9 kg to <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules* ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules* 2 through 5 years <14 kg 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules* ≥14 kg 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules* 6 through 11 years - 1 tablet of lumacaftor 100 mg/ivacaftor 125 mg* 1 tablet of lumacaftor 100 mg/ivacaftor 125 mg* 12 years and older - 1 tablet of lumacaftor 200 mg/ivacaftor 125 mg* 1 tablet of lumacaftor 200 mg/ivacaftor 125 mg* Close2.3 Dosage Adjustment for Patients Taking CYP3A Inhibitors
No dosage adjustment is necessary when CYP3A inhibitors are initiated in patients already taking ORKAMBI. However, when initiating ORKAMBI in patients currently taking strong CYP3A inhibitors, reduce the ORKAMBI dosage for the first week of treatment based on age as follows [see Dosage and Administration (2.1) and Drug Interactions (7.1)]:
- 1 through 5 years of age: 1 packet of granules every other day
- 6 years of age and older: 1 tablet daily
Following this one-week period, resume the recommended daily dosage.
If ORKAMBI is interrupted for more than one-week and then re-initiated while taking strong CYP3A inhibitors, reduce the ORKAMBI dosage for the first week of treatment re-initiation based on age as follows:
- 1 through 5 years of age: 1 packet of granules every other day
- 6 years of age and older: 1 tablet daily
Following this one-week period, resume the recommended daily dosage.
-
3 DOSAGE FORMS AND STRENGTHSTablets: 100 mg lumacaftor and 125 mg ivacaftor; supplied as pink, oval-shaped, film-coated, fixed-dose combination tablets containing 100 mg of lumacaftor and 125 mg of ivacaftor. Each tablet is ...
- Tablets: 100 mg lumacaftor and 125 mg ivacaftor; supplied as pink, oval-shaped, film-coated, fixed-dose combination tablets containing 100 mg of lumacaftor and 125 mg of ivacaftor. Each tablet is printed with the characters "1V125" in black ink on one side and plain on the other.
- Tablets: 200 mg lumacaftor and 125 mg ivacaftor; supplied as pink, oval-shaped, film-coated, fixed-dose combination tablets containing 200 mg of lumacaftor and 125 mg of ivacaftor. Each tablet is printed with the characters "2V125" in black ink on one side and plain on the other.
- Oral granules: Unit-dose packets containing lumacaftor 75 mg/ivacaftor 94 mg or lumacaftor 100 mg/ivacaftor 125 mg or lumacaftor 150 mg/ivacaftor 188 mg per packet; supplied as small, white to off-white granules in unit-dose packets.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Use in Patients with Advanced Liver Disease - Worsening of liver function, including hepatic encephalopathy, in patients with advanced liver disease has been reported. Liver function ...
5.1 Use in Patients with Advanced Liver Disease
Worsening of liver function, including hepatic encephalopathy, in patients with advanced liver disease has been reported. Liver function decompensation, including liver failure leading to death, has been reported in CF patients with pre-existing cirrhosis with portal hypertension while receiving ORKAMBI. Use ORKAMBI with caution in patients with advanced liver disease and only if the benefits are expected to outweigh the risks. If ORKAMBI is used in these patients, they should be closely monitored after the initiation of treatment and the dosage should be reduced [see Dosage and Administration (2.2) and Adverse Reactions (6.1)].
5.2 Liver-related Events
Serious adverse reactions related to elevated transaminases have been reported in patients with CF receiving ORKAMBI. In some instances, these elevations have been associated with concomitant elevations in total serum bilirubin.
It is recommended that ALT, AST, and bilirubin be assessed prior to initiating ORKAMBI, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of ALT, AST, or bilirubin elevations, more frequent monitoring should be considered. Patients who develop increased ALT, AST, or bilirubin should be closely monitored until the abnormalities resolve.
Dosing should be interrupted in patients with ALT or AST >5 × upper limit of normal (ULN) when not associated with elevated bilirubin. Dosing should also be interrupted in patients with ALT or AST elevations >3 × ULN when associated with bilirubin elevations >2 × ULN. Following resolution of transaminase elevations, consider the benefits and risks of resuming dosing [see Adverse Reactions (6.1)].
5.3 Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions, including cases of angioedema and anaphylaxis, have been reported in the postmarketing setting [see Adverse Reactions (6.2)]. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue ORKAMBI and institute appropriate therapy. Consider the benefits and risks for the individual patient to determine whether to resume treatment with ORKAMBI.
5.4 Respiratory Events
Respiratory events (e.g., chest discomfort, dyspnea, and respiration abnormal) were observed more commonly in patients during initiation of ORKAMBI compared to those who received placebo. These events have led to drug discontinuation and can be serious, particularly in patients with advanced lung disease (percent predicted FEV1 <40). Clinical experience in patients with ppFEV1 <40 is limited, and additional monitoring of these patients is recommended during initiation of therapy [see Adverse Reactions (6.1)].
5.5 Effect on Blood Pressure
Increased blood pressure has been observed in some patients treated with ORKAMBI. Blood pressure should be monitored periodically in all patients being treated with ORKAMBI [see Adverse Reactions (6.1)].
5.6 Drug Interactions
Substrates of CYP3A
Lumacaftor is a strong inducer of CYP3A. Administration of ORKAMBI may decrease systemic exposure of medicinal products that are substrates of CYP3A, which may decrease therapeutic effect. Co-administration with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index is not recommended.
ORKAMBI may substantially decrease hormonal contraceptive exposure, reducing their effectiveness and increasing the incidence of menstruation-associated adverse reactions, e.g., amenorrhea, dysmenorrhea, menorrhagia, menstrual irregular (27% in women using hormonal contraceptives compared with 3% in women not using hormonal contraceptives). Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with ORKAMBI [see Adverse Reactions (6.1), Drug Interactions (7.3, 7.11), and Clinical Pharmacology (12.3)].
Strong CYP3A Inducers
Ivacaftor is a substrate of CYP3A4 and CYP3A5 isoenzymes. Use of ORKAMBI with strong CYP3A inducers, such as rifampin, significantly reduces ivacaftor exposure, which may reduce the therapeutic effectiveness of ORKAMBI. Therefore, co-administration with strong CYP3A inducers (e.g., rifampin, St. John's wort [Hypericum perforatum]) is not recommended [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)].
Close5.7 Cataracts
Cases of non-congenital lens opacities have been reported in pediatric patients treated with ORKAMBI and ivacaftor, a component of ORKAMBI. Although other risk factors were present in some cases (such as corticosteroid use and exposure to radiation), a possible risk attributable to ivacaftor cannot be excluded [see Use in Specific Populations (8.4)]. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating ORKAMBI treatment.
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the label: Use in Patients with Advanced Liver Disease [see Warnings and Precautions (5.1)] Liver-related ...
The following adverse reactions are discussed in greater detail in other sections of the label:
- Use in Patients with Advanced Liver Disease [see Warnings and Precautions (5.1)]
- Liver-related Events [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions, Including Anaphylaxis [see Warnings and Precautions (5.3)]
- Respiratory Events [see Warnings and Precautions (5.4)]
- Effect on Blood Pressure [see Warnings and Precautions (5.5)]
- Cataracts [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The overall safety profile of ORKAMBI is based on the pooled data from 1108 patients with CF aged 12 years and older who are homozygous for the F508del mutation in the CFTR gene and who received at least one dose of study drug in two double-blind, placebo-controlled, Phase 3 clinical trials, each with 24 weeks of treatment (Trials 1 and 2).
In addition, the following clinical trials have been conducted:
- A 24-week, open-label trial (Trial 3) in 58 patients with CF aged 6 through 11 years homozygous for the F508del-CFTR mutation.
- A 24-week, placebo-controlled trial (Trial 4) in 204 patients aged 6 through 11 years homozygous for the F508del-CFTR mutation.
- A 24-week, open-label trial (Trial 5) in 46 patients aged 12 years and older homozygous for the F508del-CFTR mutation and with advanced lung disease (ppFEV1 <40).
- A 24-week, open-label trial (Trial 6) in 60 patients aged 2 through 5 years homozygous for the F508del-CFTR mutation.
- A 24-week, open-label trial (Trial 7) in 46 patients aged 1 through 2 years homozygous for the F508del-CFTR mutation.
Of the 1108 patients, in the pooled analyses of Trial 1 and Trial 2, 49% were female and 99% were Caucasian; 369 patients received ORKAMBI every 12 hours and 370 patients received placebo.
The proportion of patients who prematurely discontinued study drug due to adverse events was 5% for patients treated with ORKAMBI and 2% for patients who received placebo.
Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in patients treated with ORKAMBI included pneumonia, hemoptysis, cough, increased blood creatine phosphokinase, and transaminase elevations. These occurred in 1% or less of patients.
Table 3 shows adverse reactions occurring in ≥5% of patients with CF aged 12 years and older treated with ORKAMBI who are homozygous for the F508del mutation in the CFTR gene that also occurred at a higher rate than in patients who received placebo in the two double-blind, placebo-controlled trials.
Table 3: Incidence of Adverse Drug Reactions in ≥5% of ORKAMBI-Treated Patients Aged 12 Years and Older Who are Homozygous for the F508del Mutation in the CFTR Gene in 2 Placebo-Controlled Phase 3 Clinical Trials of 24 Weeks Duration Adverse Reaction
(Preferred Term)ORKAMBI
N=369
(%)Placebo
N=370
(%)Dyspnea 48 (13) 29 (8) Nasopharyngitis 48 (13) 40 (11) Nausea 46 (13) 28 (8) Diarrhea 45 (12) 31 (8) Upper respiratory tract infection 37 (10) 20 (5) Fatigue 34 (9) 29 (8) Respiration abnormal 32 (9) 22 (6) Blood creatine phosphokinase increased 27 (7) 20 (5) Rash 25 (7) 7 (2) Flatulence 24 (7) 11 (3) Rhinorrhea 21 (6) 15 (4) Influenza 19 (5) 8 (2) The safety profile from two pediatric trials in CF patients aged 6 through 11 years who are homozygous for the F508del-CFTR mutation, a 24-week, open-label, multicenter safety trial in 58 patients (Trial 3) and a 24-week, placebo-controlled, clinical trial (Trial 4) in 204 patients (103 received lumacaftor 200 mg/ivacaftor 250 mg every 12 hours and 101 received placebo), was similar to that observed in Trials 1 and 2. Adverse reactions that are not listed in Table 3, and that occurred in ≥5% of lumacaftor/ivacaftor-treated patients with an incidence of ≥3% higher than placebo included: productive cough (17.5% vs 5.9%), nasal congestion (16.5% vs 7.9%), headache (12.6% vs 8.9%), abdominal pain upper (12.6% vs 6.9%), and sputum increased (10.7% vs 2.0%).
In a 24-week, open-label, multicenter, study in 60 patients aged 2 through 5 years with CF who were homozygous for the F508del-CFTR mutation (Trial 6) the safety profile was similar to that observed in studies in patients aged 6 years and older [see Clinical Pharmacology (12.2)].
In a 24-week, open-label, multicenter, study in 46 patients aged 1 through 2 years with CF who were homozygous for the F508del-CFTR mutation (Trial 7) the safety profile was similar to that observed in studies in patients aged 2 years and older [see Clinical Pharmacology (12.2)].
Additional information on selected adverse reactions from trials is detailed below:
Description of Selected Adverse Drug Reactions
Liver-related Adverse Reactions
In Trials 1 and 2, the incidence of maximum transaminase (ALT or AST) levels >8, >5, and >3 × ULN elevations were similar between patients treated with ORKAMBI and those who received placebo. Three patients who received ORKAMBI had liver-related serious adverse reactions, including two reported as transaminase elevations and one as hepatic encephalopathy, compared to none in the placebo group. Of these three, one had elevated transaminases (>3 × ULN) associated with bilirubin elevation >2 × ULN. Following discontinuation or interruption of ORKAMBI, transaminases decreased to <3 × ULN.
Among six patients with pre-existing cirrhosis and/or portal hypertension who received ORKAMBI, worsening liver function with increased ALT, AST, bilirubin, and hepatic encephalopathy was observed in one patient. The event occurred within five days of the start of dosing and resolved following discontinuation of ORKAMBI [see Warnings and Precautions (5.1, 5.2)].
During the 24-week, open-label, clinical trial in 58 patients aged 6 through 11 years (Trial 3), the incidence of maximum transaminase (ALT or AST) levels >8, >5, and >3 × ULN was 5%, 9%, and 19%. No patients had total bilirubin levels >2 × ULN. Lumacaftor/ivacaftor dosing was maintained or successfully resumed after interruption in all patients with transaminase elevations, except one patient who discontinued treatment permanently.
During the 24-week, placebo-controlled, clinical trial in 204 patients aged 6 through 11 years (Trial 4), the incidence of maximum transaminase (ALT or AST) levels >8, >5, and >3 × ULN was 1%, 5%, and 13% in the lumacaftor/ivacaftor patients, and 2%, 3%, and 8% in the placebo-treated patients. No patients had total bilirubin levels >2 × ULN. Two patients in the lumacaftor/ivacaftor group and two patients in the placebo group discontinued treatment permanently due to transaminase elevations.
During the 24-week, open-label, clinical trial in 60 patients aged 2 through 5 years (Trial 6), the incidence of maximum transaminase (ALT or AST) levels >8, >5, and >3 × ULN was 8.3% (5/60), 11.7% (7/60), and 15.0% (9/60). No patients had total bilirubin levels >2 × ULN. Three patients discontinued lumacaftor/ivacaftor treatment permanently due to transaminase elevations.
During the 24-week, open-label, clinical trial in 46 patients aged 1 through 2 years (Trial 7), the incidence of maximum transaminase (ALT or AST) levels >8, >5, and >3 × ULN was 2.2% (1/46), 4.3% (2/46), and 10.9% (5/46), respectively. No patients had total bilirubin levels >2 × ULN. One patient discontinued lumacaftor/ivacaftor treatment permanently due to transaminase elevations.
Respiratory Adverse Reactions
In Trials 1 and 2, the incidence of respiratory symptom-related adverse reactions (e.g., chest discomfort, dyspnea, and respiration abnormal) was more common in patients treated with ORKAMBI (22%) compared to patients who received placebo (14%). The incidence of these adverse reactions was more common in patients treated with ORKAMBI with lower pre-treatment FEV1. In patients treated with ORKAMBI, the majority of the events began during the first week of treatment [see Warnings and Precautions (5.4)].
During the 24-week, open-label, clinical trial in 46 patients aged 12 years and older (Trial 5) with advanced lung disease (ppFEV1 <40) [mean ppFEV1 29.1 at baseline (range: 18.3 to 42.0)], the incidence of respiratory symptom-related adverse reactions was 65% [see Warnings and Precautions (5.4)].
During the 24-week, open-label, clinical trial (Trial 3) in 58 patients aged 6 through 11 years (mean baseline ppFEV1 was 91.4), the incidence of respiratory symptom-related adverse reactions was 3% (2/58).
During the 24-week, placebo-controlled, clinical trial (Trial 4) in patients aged 6 through 11 years [mean ppFEV1 89.8 at baseline (range: 48.6 to 119.6)], the incidence of respiratory symptom-related adverse reactions was 11% in lumacaftor/ivacaftor patients and 9% in placebo patients. A decline in ppFEV1 at initiation of therapy was observed during serial post-dose spirometry assessments. The absolute change from pre-dose at 4-6 hours post-dose was -7.7 on Day 1 and -1.3 on Day 15 in lumacaftor/ivacaftor patients. The post-dose decline was resolved by Week 16.
Menstrual Abnormalities
In Trials 1 and 2, the incidence of combined menstrual abnormality adverse reactions (e.g., amenorrhea, dysmenorrhea, menorrhagia, menstrual irregular) was more common in female patients treated with ORKAMBI (10%) compared to placebo (2%). These events occurred more frequently in the subset of female patients treated with ORKAMBI who were using hormonal contraceptives (27%) compared to those not using hormonal contraceptives (3%) [see Warnings and Precautions (5.6) and Drug Interactions (7.11)].
Increased Blood Pressure
In Trials 1 and 2, adverse reactions related to increases in blood pressure (e.g., hypertension, blood pressure increased) were reported in 1.1% (4/369) of patients treated with ORKAMBI and in no patients who received placebo.
The proportion of patients who experienced a systolic blood pressure value >140 mmHg or a diastolic blood pressure >90 mmHg on at least two occasions was 3.6% and 2.2% in patients treated with ORKAMBI, respectively, compared with 1.6% and 0.5% in patients who received placebo [see Warnings and Precautions (5.5)].
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ORKAMBI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepatobiliary: liver function decompensation including liver failure leading to death in patients with pre-existing cirrhosis with portal hypertension [see Warnings and Precautions (5.1)].
Immune system disorders: anaphylaxis, angioedema
-
7 DRUG INTERACTIONSPotential for Other Drugs to Affect Lumacaftor/Ivacaftor - 7.1 Inhibitors of CYP3A - Co-administration of lumacaftor/ivacaftor with itraconazole, a strong CYP3A inhibitor, did not impact the ...
7.1 Inhibitors of CYP3A
Co-administration of lumacaftor/ivacaftor with itraconazole, a strong CYP3A inhibitor, did not impact the exposure of lumacaftor, but increased ivacaftor exposure by 4.3-fold. Due to the induction effect of lumacaftor on CYP3A, at steady-state, the net exposure of ivacaftor is not expected to exceed that when given in the absence of lumacaftor at a dose of 150 mg every 12 hours (the approved dose of ivacaftor monotherapy). Therefore, no dosage adjustment is necessary when CYP3A inhibitors are initiated in patients currently taking ORKAMBI. However, when initiating ORKAMBI in patients taking strong CYP3A inhibitors, reduce the ORKAMBI dosage as recommended for the first week of treatment to allow for the steady-state induction effect of lumacaftor. Following this period, continue with the recommended daily dose [see Dosage and Administration (2.3)].
Examples of strong CYP3A inhibitors include:
- ketoconazole, itraconazole, posaconazole, and voriconazole.
- telithromycin, clarithromycin.
No dosage adjustment is recommended when used with moderate or weak CYP3A inhibitors.
7.2 Inducers of CYP3A
Co-administration of lumacaftor/ivacaftor with rifampin, a strong CYP3A inducer, had minimal effect on the exposure of lumacaftor, but decreased ivacaftor exposure (AUC) by 57%. This may reduce the effectiveness of ORKAMBI. Therefore, co-administration with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John's wort (Hypericum perforatum), is not recommended [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
No dosage adjustment is recommended when used with moderate or weak CYP3A inducers.
7.3 CYP3A Substrates
Lumacaftor is a strong inducer of CYP3A. Co-administration of lumacaftor with ivacaftor, a sensitive CYP3A substrate, decreased ivacaftor exposure by approximately 80%. Administration of ORKAMBI may decrease systemic exposure of medicinal products which are substrates of CYP3A, thereby decreasing the therapeutic effect of the medicinal product.
Co-administration of ORKAMBI is not recommended with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)] such as:
- Benzodiazepines: midazolam, triazolam (consider an alternative to these benzodiazepines).
- Immunosuppressants: cyclosporine, everolimus, sirolimus, and tacrolimus (avoid the use of ORKAMBI).
7.4 CYP2B6 and CYP2C Substrates
In vitro studies suggest that lumacaftor has the potential to induce CYP2B6, CYP2C8, CYP2C9, and CYP2C19; inhibition of CYP2C8 and CYP2C9 has also been observed in vitro. Additionally, in vitro studies suggest that ivacaftor may inhibit CYP2C9. Therefore, concomitant use of ORKAMBI with CYP2B6, CYP2C8, CYP2C9, and CYP2C19 substrates may alter the exposure of these substrates.
7.5 Digoxin and Other P-gp Substrates
Based on in vitro results which showed P-gp inhibition and pregnane-X-receptor (PXR) activation, lumacaftor has the potential to both inhibit and induce P-gp. Additionally, a clinical study with ivacaftor monotherapy showed that ivacaftor is a weak inhibitor of P-gp. Therefore, concomitant use of ORKAMBI with P-gp substrates may alter the exposure of these substrates.
Monitor the serum concentration of digoxin and titrate the digoxin dose to obtain the desired clinical effect.
7.6 Anti-allergics and Systemic Corticosteroids
ORKAMBI may decrease the exposure of montelukast, which may reduce its efficacy. No dosage adjustment for montelukast is recommended. Employ appropriate clinical monitoring, as is reasonable, when co-administered with ORKAMBI.
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of prednisone and methylprednisolone. A higher dose of these systemic corticosteroids may be required to obtain the desired clinical effect.
7.7 Antibiotics
Concomitant use of ORKAMBI may decrease the exposure of clarithromycin, erythromycin, and telithromycin, which may reduce the effectiveness of these antibiotics. Consider an alternative to these antibiotics, such as ciprofloxacin, azithromycin, and levofloxacin.
7.8 Antifungals
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of itraconazole, ketoconazole, posaconazole, and voriconazole. Concomitant use of ORKAMBI with these antifungals is not recommended. Monitor patients closely for breakthrough fungal infections if such drugs are necessary. Consider an alternative such as fluconazole.
7.9 Anti-inflammatories
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of ibuprofen. A higher dose of ibuprofen may be required to obtain the desired clinical effect.
7.10 Antidepressants
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of citalopram, escitalopram, and sertraline. A higher dose of these antidepressants may be required to obtain the desired clinical effect.
7.11 Hormonal Contraceptives
ORKAMBI may decrease hormonal contraceptive exposure, reducing the effectiveness. Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with ORKAMBI.
Concomitant use of ORKAMBI with hormonal contraceptives increased the menstrual abnormality events [see Adverse Reactions (6.1)]. Avoid concomitant use unless the benefit outweighs the risks.
7.12 Oral Hypoglycemics
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of repaglinide and may alter the exposure of sulfonylurea. A dosage adjustment may be required to obtain the desired clinical effect. No dosage adjustment is recommended for metformin.
7.13 Proton Pump Inhibitors, H2 Blockers, Antacids
ORKAMBI may reduce the exposure and effectiveness of proton pump inhibitors such as omeprazole, esomeprazole, and lansoprazole, and may alter the exposure of ranitidine. A dose adjustment may be required to obtain the desired clinical effect. No dose adjustment is recommended for calcium carbonate antacid.
7.14 Warfarin
ORKAMBI may alter the exposure of warfarin. Monitor the international normalized ratio (INR) when warfarin co-administration with ORKAMBI is required.
Close7.15 Concomitant Drugs That Do Not Need Dosage Adjustment
No dosage adjustment of ORKAMBI or concomitant drug is recommended when ORKAMBI is given with the following: azithromycin, aztreonam, budesonide, ceftazidime, cetirizine, ciprofloxacin, colistimethate, colistin, dornase alfa, fluticasone, ipratropium, levofloxacin, pancreatin, pancrelipase, salbutamol, salmeterol, sulfamethoxazole, trimethoprim, tiotropium, and tobramycin. Based on the metabolism and route of elimination, ORKAMBI is not expected to impact the exposure of these drugs.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are limited and incomplete human data from clinical trials and postmarketing reports on use of ORKAMBI or its individual components, lumacaftor or ...
8.1 Pregnancy
Risk Summary
There are limited and incomplete human data from clinical trials and postmarketing reports on use of ORKAMBI or its individual components, lumacaftor or ivacaftor, in pregnant women to inform a drug-associated risk. In animal reproduction studies, oral administration of lumacaftor to pregnant rats and rabbits during organogenesis demonstrated no adverse effects on fetal development at doses that produced maternal exposures up to approximately 8 (rats) and 5 (rabbits) times the exposure at the maximum recommended human dose (MRHD). Oral administration of ivacaftor to pregnant rats and rabbits during organogenesis demonstrated no adverse effects on fetal development at doses that produced maternal exposures up to approximately 7 (rats) and 45 (rabbits) times the exposure at the MRHD. No adverse developmental effects were observed after oral administration of either lumacaftor or ivacaftor to pregnant rats from organogenesis through lactation at doses that produced maternal exposures approximately 8 and 5 times the exposures at the MRHD, respectively (see Data). There are no animal reproduction studies with concomitant administration of lumacaftor and ivacaftor.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Lumacaftor
In an embryo-fetal development (EFD) study, pregnant rats were administered lumacaftor at oral doses of 500, 1000, or 2000 mg/kg/day during the period of organogenesis from gestation days 7-17. Lumacaftor did not affect fetal development or survival at exposures up to 8 times the MRHD (on an AUC basis at maternal oral doses up to 2000 mg/kg/day). In an EFD study, pregnant rabbits were administered lumacaftor at oral doses of 50, 100, or 200 mg/kg/day during the period of organogenesis from gestation days 7-19. Lumacaftor did not affect fetal development or survival at exposures up to 5 times the MRHD (on an AUC basis at maternal oral doses up to 200 mg/kg/day). Maternal toxicity as evidenced by decreased body weight, decreased food consumption, and clinical signs was observed at 100 and 200 mg/kg/day without any adverse fetal effects. In a pre- and post-natal development study in pregnant female rats administered lumacaftor at 250, 500, or 1000 mg/kg/day from gestation day 6 through lactation day 20, lumacaftor had no effects on delivery or growth and development of offspring at exposures up to 8 times the MRHD (on an AUC basis at maternal oral doses up to 1000 mg/kg/day). Placental transfer of lumacaftor was observed in pregnant rats and rabbits.
Ivacaftor
In an EFD study, pregnant rats were administered ivacaftor at oral doses of 50, 100, or 200 mg/kg/day during the period of organogenesis from gestation days 7-17. Ivacaftor did not affect fetal survival at exposures up to 7 times the MRHD (based on summed AUCs for ivacaftor and its metabolites at maternal oral doses up to 200 mg/kg/day). Maternal toxicity (i.e., decreased mean body weight and body weight gain) was observed at 100 and 200 mg/kg/day (5 and 7 times the exposure at the MRHD, respectively) and was associated with a decrease in fetal body weights at a maternal dose of 200 mg/kg/day (7 times the MRHD). In an EFD study, pregnant rabbits were administered ivacaftor at oral doses of 25, 50, or 100 mg/kg/day during the period of organogenesis from gestation days 7-19. Ivacaftor did not affect fetal development or survival at exposures up to 45 times the MRHD (on an ivacaftor AUC basis at maternal oral doses up to 100 mg/kg/day). Maternal toxicity (i.e., death, decreased food consumption, decreased mean body weight and body weight gain, decreased clinical condition, abortions) was observed at doses greater than or equal to 50 mg/kg/day (approximately 15 times the MRHD). In a pre- and post-natal development study, pregnant rats were administered ivacaftor at oral doses of 50, 100, or 200 mg/kg/day from gestation day 7 through lactation day 20. Ivacaftor had no effects on delivery or growth and development of offspring at exposures up to 5 times the MRHD (based on summed AUCs for ivacaftor and its metabolites at maternal oral doses up to 100 mg/kg/day). Decreased fetal body weights were observed at a maternally toxic dose that produced exposures 7 times the MRHD (based on summed AUCs for ivacaftor and its metabolites at a maternal oral dose of 200 mg/kg/day). Placental transfer of ivacaftor was observed in pregnant rats and rabbits.
8.2 Lactation
Risk Summary
There is no information regarding the presence of lumacaftor or ivacaftor in human milk, the effects on the breastfed infant, or the effects on milk production. Both lumacaftor and ivacaftor are excreted into the milk of lactating rats; however, due to species-specific differences in lactation physiology, animal lactation data may not reliably predict levels in human milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ORKAMBI and any potential adverse effects on the breastfed child from ORKAMBI or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
ORKAMBI may decrease hormonal contraceptive exposure, reducing the effectiveness. Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with ORKAMBI [see Warnings and Precautions (5.6) and Drug Interactions (7.11)].
8.4 Pediatric Use
The safety and effectiveness of ORKAMBI in pediatric patients aged one year and older have been established. Use of ORKAMBI in these age groups is supported by evidence from adequate and well-controlled studies of ORKAMBI in patients aged 12 years and older [see Clinical Studies (14) and Adverse Reactions (6.1)] with additional data as follows:
- Extrapolation of efficacy in patients aged 12 years and older homozygous for the F508del mutation in the CFTR gene to pediatric patients aged 1 through 11 years with support from population pharmacokinetic analyses showing similar drug exposure levels in patients aged 12 years and older and in patients aged 1 through 11 years [see Clinical Pharmacology (12.3)].
- Safety data were obtained from a 24-week, open-label, clinical trial in 58 patients aged 6 through 11 years, mean age 9 years (Trial 3) and a 24-week, placebo-controlled, clinical trial in 204 patients aged 6 through 11 years (Trial 4). Trial 3 evaluated subjects with a screening ppFEV1 ≥40 [mean ppFEV1 91.4 at baseline (range: 55 to 122.7)]. Trial 4 evaluated subjects with a screening ppFEV1 ≥70 [mean ppFEV1 89.8 at baseline (range: 48.6 to 119.6)]. The safety profile of ORKAMBI in pediatric patients 6 through 11 years of age was similar to that in patients aged 12 years and older [see Adverse Reactions (6.1)]. In Trial 3, spirometry (ppFEV1) was assessed as a planned safety endpoint. The within-group LS mean absolute change from baseline in ppFEV1 at Week 24 was 2.5 percentage points. At the Week 26 safety follow-up visit (following a planned discontinuation) ppFEV1 was also assessed. The within-group LS mean absolute change in ppFEV1 from Week 24 at Week 26 was -3.2 percentage points.
- Additional safety data were obtained from Trial 6, a 24-week, open-label, clinical trial in 60 patients aged 2 through 5 years at screening (mean age at baseline 3.7 years). The safety profile in Trial 6 was similar to that in patients aged 6 years and older [see Adverse Reactions (6.1)].
- Additional safety data were obtained from Trial 7, a 24-week, open-label, clinical trial in 46 patients aged 1 to 2 years at screening (mean age at baseline 18.1 months). The safety profile in Trial 7 was similar to that in patients aged 2 years and older [see Adverse Reactions (6.1)].
- Safety was evaluated in a 96-week open-label clinical trial (Trial 8) in 52 patients (39 rolled over from Trial 7 and 13 ORKAMBI naïve) aged 1 to 2 years. Adverse reactions from trial 8 were generally similar to those reported in Trial 7.
The safety and effectiveness of ORKAMBI in patients with CF younger than 1 year of age have not been established.
Cases of non-congenital lens opacities have been reported in pediatric patients treated with ORKAMBI and ivacaftor, a component of ORKAMBI. Although other risk factors were present in some cases (such as corticosteroid use and exposure to radiation), a possible risk attributable to ivacaftor cannot be excluded [see Warnings and Precautions (5.7)].
Juvenile Animal Toxicity Data
In a juvenile toxicology study in which ivacaftor was administered to rats from post-natal days 7 to 35, cataracts were observed at all dose levels, ranging from 0.3 to 2 times the MRHD (based on summed AUCs for ivacaftor and its metabolites at oral doses of 10-50 mg/kg/day). This finding has not been observed in older animals.
8.5 Geriatric Use
CF is largely a disease of children and young adults. Clinical trials of ORKAMBI did not include sufficient numbers of patients 65 years of age and over to determine whether they respond differently from younger patients.
8.6 Hepatic Impairment
No dosage adjustment is necessary for patients with mild hepatic impairment (Child-Pugh Class A). A dose reduction to 2 tablets in the morning and 1 tablet in the evening is recommended for patients aged 6 years and older with moderate hepatic impairment (Child-Pugh Class B). A dose reduction to 1 packet of oral granules in the morning daily and 1 packet of oral granules in the evening every other day is recommended for patients aged 1 to 5 years old with moderate hepatic impairment (Child-Pugh Class B).
Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a maximum dose of 1 tablet in the morning and 1 tablet in the evening or less frequently, or 1 packet of oral granules once daily or less frequently in patients with severe hepatic impairment after weighing the risks and benefits of treatment [see Warnings and Precautions (5.1), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
8.7 Renal Impairment
ORKAMBI has not been studied in patients with mild, moderate, or severe renal impairment or in patients with end-stage renal disease. No dosage adjustment is necessary for patients with mild to moderate renal impairment. Caution is recommended while using ORKAMBI in patients with severe renal impairment (creatinine clearance ≤30 mL/min) or end-stage renal disease.
8.8 Patients with Severe Lung Dysfunction
The Phase 3 trials (Trials 1 and 2 [see Clinical Studies (14)]) included 29 patients receiving ORKAMBI with ppFEV1 <40 at baseline. The treatment effect in this subgroup was comparable to that observed in patients with ppFEV1 ≥40.
Close8.9 Patients After Organ Transplantation
ORKAMBI has not been studied in patients with CF who have undergone organ transplantation. Use in transplanted patients is not recommended due to potential drug-drug interactions [see Drug Interactions (7.3)].
-
10 OVERDOSAGEThere have been no reports of overdose with ORKAMBI. The highest repeated dose was lumacaftor 1000 mg once daily/ivacaftor 450 mg q12h administered to 49 healthy subjects for 7 days in a trial ...
There have been no reports of overdose with ORKAMBI.
The highest repeated dose was lumacaftor 1000 mg once daily/ivacaftor 450 mg q12h administered to 49 healthy subjects for 7 days in a trial evaluating the effect of ORKAMBI on electrocardiograms (ECGs). Adverse events reported at an increased incidence of ≥5% compared to the lumacaftor 600 mg/ivacaftor 250 mg dosing period and placebo included: headache (29%), transaminase increased (18%), and generalized rash (10%).
No specific antidote is available for overdose with ORKAMBI. Treatment of overdose consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
Close -
11 DESCRIPTIONThe active ingredients in ORKAMBI tablets are lumacaftor, which has the following chemical name ...
The active ingredients in ORKAMBI tablets are lumacaftor, which has the following chemical name: 3-[6-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-3-methylpyridin-2-yl]benzoic acid, and ivacaftor, a CFTR potentiator, which has the following chemical name: N-(2,4-di-tert-butyl-5-hydroxyphenyl)-1,4-dihydro-4-oxoquinoline-3-carboxamide. The molecular formula for lumacaftor is C24H18F2N2O5 and for ivacaftor is C24H28N2O3. The molecular weights for lumacaftor and ivacaftor are 452.41 and 392.49, respectively. The structural formulas are:
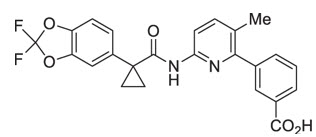
lumacaftor
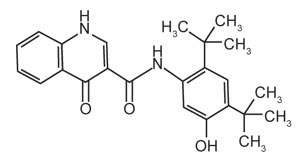
ivacaftor
Lumacaftor is a white to off-white powder that is practically insoluble in water (0.02 mg/mL). Ivacaftor is a white to off-white powder that is practically insoluble in water (<0.05 µg/mL).
ORKAMBI is available as a pink, oval-shaped, film-coated tablet for oral administration containing 200 mg of lumacaftor and 125 mg of ivacaftor. Each ORKAMBI tablet contains 200 mg of lumacaftor and 125 mg of ivacaftor, and the following inactive ingredients: cellulose, microcrystalline; croscarmellose sodium; hypromellose acetate succinate; magnesium stearate; povidone; and sodium lauryl sulfate. The tablet film coat contains carmine, FD&C Blue #1, FD&C Blue #2, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. The printing ink contains ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
ORKAMBI is also available as a pink, oval-shaped, film-coated tablet for oral administration containing 100 mg of lumacaftor and 125 mg of ivacaftor. Each ORKAMBI tablet contains 100 mg of lumacaftor and 125 mg of ivacaftor, and the following inactive ingredients: cellulose, microcrystalline; croscarmellose sodium; hypromellose acetate succinate; magnesium stearate; povidone; and sodium lauryl sulfate. The tablet film coat contains carmine, FD&C Blue #1, FD&C Blue #2, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. The printing ink contains ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
ORKAMBI is also available as white to off-white granules for oral administration and enclosed in a unit-dose packet containing lumacaftor 75 mg/ivacaftor 94 mg, lumacaftor 100 mg/ivacaftor 125 mg or lumacaftor 150 mg/ivacaftor 188 mg per packet. Each unit-dose packet of ORKAMBI oral granules contains lumacaftor 75 mg/ivacaftor 94 mg, lumacaftor 100 mg/ivacaftor 125 mg or lumacaftor 150 mg/ivacaftor 188 mg per packet and the following inactive ingredients: cellulose, microcrystalline; croscarmellose sodium; hypromellose acetate succinate; povidone; and sodium lauryl sulfate.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The CFTR protein is a chloride channel present at the surface of epithelial cells in multiple organs. The F508del mutation results in protein misfolding, causing a ...
12.1 Mechanism of Action
The CFTR protein is a chloride channel present at the surface of epithelial cells in multiple organs. The F508del mutation results in protein misfolding, causing a defect in cellular processing and trafficking that targets the protein for degradation and therefore reduces the quantity of CFTR at the cell surface. The small amount of F508del-CFTR that reaches the cell surface is less stable and has low channel-open probability (defective gating activity) compared to wild-type CFTR protein.
Lumacaftor improves the conformational stability of F508del-CFTR, resulting in increased processing and trafficking of mature protein to the cell surface. Ivacaftor is a CFTR potentiator that facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the CFTR protein at the cell surface. In vitro studies have demonstrated that both lumacaftor and ivacaftor act directly on the CFTR protein in primary human bronchial epithelial cultures and other cell lines harboring the F508del-CFTR mutation to increase the quantity, stability, and function of F508del-CFTR at the cell surface, resulting in increased chloride ion transport. In vitro responses do not necessarily correspond to in vivo pharmacodynamic response or clinical benefit.
12.2 Pharmacodynamics
Sweat Chloride Evaluation
Changes in sweat chloride in response to relevant doses of lumacaftor alone or in combination with ivacaftor were evaluated in a double-blind, placebo-controlled, Phase 2 clinical trial in patients with CF aged 18 years and older either homozygous or heterozygous for the F508del mutation. In that trial, 10 patients (homozygous for F508del) completed dosing with lumacaftor alone 400 mg q12h for 28 days followed by the addition of ivacaftor 250 mg q12h for an additional 28 days and 25 patients (homozygous or heterozygous for F508del) completed dosing with placebo. The treatment difference between lumacaftor 400 mg q12h alone and placebo evaluated as mean change in sweat chloride from baseline to Day 28 compared to placebo was -8.2 mmol/L (95% CI: -14, -2). The treatment difference between the combination of lumacaftor 400 mg/ivacaftor 250 mg q12h and placebo evaluated as mean change in sweat chloride from baseline to Day 56 compared to placebo was -11 mmol/L (95% CI: -18, -4).
Changes in sweat chloride in response to lumacaftor/ivacaftor were also evaluated in a 24-week, open-label, clinical trial (Trial 3) in 58 patients with CF, aged 6 through 11 years (homozygous for F508del) who received lumacaftor 200 mg/ivacaftor 250 mg q12h for 24 weeks. Patients treated with lumacaftor/ivacaftor had a reduction in sweat chloride at Day 15 that was sustained through Week 24. The within-group LS mean absolute change from baseline in sweat chloride was -20.4 mmol/L at Day 15 and -24.8 mmol/L at Week 24. In addition, sweat chloride was also assessed after a 2-week washout period to evaluate the off-drug response. The within-group LS mean absolute change in sweat chloride from Week 24 at Week 26 following the 2-week washout period was 21.3 mmol/L.
Changes in sweat chloride in response to lumacaftor/ivacaftor were also evaluated in a 24-week, open-label, clinical trial (Trial 6) in 60 patients with CF, aged 2 through 5 years (homozygous for F508del) who received either lumacaftor 100 mg/ivacaftor 125 mg every 12 hours or lumacaftor 150 mg/ivacaftor 188 mg every 12 hours for 24 weeks. Treatment with lumacaftor/ivacaftor demonstrated a reduction in sweat chloride at Week 4 that was sustained through Week 24. The mean absolute change from baseline in sweat chloride was –31.7 mmol/L (95% CI: -35.7, -27.6) at Week 24. In addition, sweat chloride was also assessed after a 2-week washout period to evaluate the off-drug response. The mean absolute change in sweat chloride from Week 24 at Week 26 following the 2-week washout period was an increase of 33.0 mmol/L (95% CI: 28.9, 37.1; P<0.0001).
Changes in sweat chloride in response to lumacaftor/ivacaftor were evaluated in a 24-week, open-label, clinical trial (Trial 7) in 46 patients with CF, aged 1 through 2 years (homozygous for F508del) who received lumacaftor 75 mg/ivacaftor 94 mg (patient weighing 7 kg to <9 kg at screening), lumacaftor 100 mg/ivacaftor 125 mg (patient weighing 9 kg to <14 kg at screening), lumacaftor 150 mg/ivacaftor 188 mg (patient weighing ≥14 kg at screening), every 12 hours for 24 weeks. Treatment with lumacaftor/ivacaftor demonstrated a reduction in sweat chloride at Week 4 which was sustained through Week 24. The mean absolute change from baseline in sweat chloride at Week 24 was -29.1 mmol/L (95% CI: -34.8, -23.4). In addition, sweat chloride was also assessed after a 2-week washout period to evaluate the off-drug response. The mean absolute change in sweat chloride from Week 24 at Week 26 following the 2-week washout period was 27.3 mmol/L (95% CI: 22.3, 32.3).
There was no direct correlation between decrease in sweat chloride levels and improvement in lung function (ppFEV1).
Cardiac Electrophysiology
The effect of multiple doses of lumacaftor 600 mg once daily/ivacaftor 250 mg q12h and lumacaftor 1000 mg once daily/ivacaftor 450 mg q12h on QTc interval was evaluated in a randomized, placebo- and active-controlled (400 mg moxifloxacin), parallel, thorough QT study in 168 healthy subjects. No meaningful changes in QTc interval were observed with either lumacaftor 600 mg once daily/ivacaftor 250 mg q12h and lumacaftor 1000 mg once daily/ivacaftor 450 mg q12h dose groups. A maximum decrease in mean heart rate of up to 8 beats per minute (bpm) from baseline was observed with lumacaftor/ivacaftor treatment. In Trials 1 and 2, a similar decrease in heart rate was observed in patients during initiation of ORKAMBI (lumacaftor 400 mg/ivacaftor 250 mg q12h).
Close12.3 Pharmacokinetics
The exposure (AUC) of lumacaftor is approximately 2-fold higher in healthy adult volunteers compared to exposure in patients with CF. The exposure of ivacaftor is similar between healthy adult volunteers and patients with CF. After twice daily dosing, steady-state plasma concentrations of lumacaftor and ivacaftor in healthy subjects were generally reached after approximately 7 days of treatment, with an accumulation ratio of approximately 1.9 for lumacaftor. The steady-state exposure of ivacaftor is lower than that of Day 1 due to the CYP3A induction effect of lumacaftor.
Table 4: Mean (SD) Pharmacokinetic Parameters of Lumacaftor and Ivacaftor at Steady-State in Subjects with CF Drug Cmax
(μg/mL)t½*
(h)AUC0-12h
(μg∙h/mL)- *
- Based on lumacaftor 200 mg q12h/ivacaftor 250 mg q12h studied in healthy subjects.
Lumacaftor 400 mg q12h/
Ivacaftor 250 mg q12hLumacaftor 25.0 (7.96) 25.2 (9.94) 198 (64.8) Ivacaftor 0.602 (0.304) 9.34 (3.81) 3.66 (2.25) Absorption
When a single dose of lumacaftor/ivacaftor was administered with fat-containing foods, lumacaftor exposure was approximately 2 times higher and ivacaftor exposure was approximately 3 times higher than when taken in a fasting state.
Following multiple oral dose administration of lumacaftor in combination with ivacaftor, the exposure of lumacaftor generally increased proportional to dose over the range of 200 mg every 24 hours to 400 mg every 12 hours. The median (range) tmax of lumacaftor is approximately 4.0 hours (2.0; 9.0) in the fed state.
Following multiple oral dose administration of ivacaftor in combination with lumacaftor, the exposure of ivacaftor generally increased with dose from 150 mg every 12 hours to 250 mg every 12 hours. The median (range) tmax of ivacaftor is approximately 4.0 hours (2.0; 6.0) in the fed state.
Distribution
Lumacaftor is approximately 99% bound to plasma proteins, primarily to albumin. After oral administration of 200 mg every 24 hours for 28 days to patients with CF in a fed state, the mean (±SD) for apparent volumes of distribution was 86.0 (69.8) L.
Ivacaftor is approximately 99% bound to plasma proteins, primarily to alpha 1-acid glycoprotein and albumin.
Elimination
The half-life of lumacaftor is approximately 26 hours in patients with CF. The typical apparent clearance, CL/F (CV), of lumacaftor was estimated to be 2.38 L/hr (29.4%) for patients with CF. The half-life of ivacaftor when given with lumacaftor is approximately 9 hours in healthy subjects. The typical CL/F (CV), of ivacaftor when given in combination with lumacaftor was estimated to be 25.1 L/hr (40.5%) for patients with CF.
Metabolism
Lumacaftor is not extensively metabolized in humans with the majority of lumacaftor excreted unchanged in the feces. In vitro and in vivo data indicate that lumacaftor is mainly metabolized via oxidation and glucuronidation.
Ivacaftor is extensively metabolized in humans. In vitro and in vivo data indicate that ivacaftor is primarily metabolized by CYP3A. M1 and M6 are the two major metabolites of ivacaftor in humans.
Excretion
Following oral administration of lumacaftor, the majority of lumacaftor (51%) is excreted unchanged in the feces. There was minimal elimination of lumacaftor and its metabolites in urine (only 8.6% of total radioactivity was recovered in the urine with 0.18% as unchanged parent).
Following oral administration of ivacaftor alone, the majority of ivacaftor (87.8%) is eliminated in the feces after metabolic conversion. There was minimal elimination of ivacaftor and its metabolites in urine (only 6.6% of total radioactivity was recovered in the urine).
Specific Populations
Pediatric Patients
The following conclusions about exposures between adults and the pediatric population are based on population pharmacokinetics (PK) analyses:
Table 5: Mean (SD) Lumacaftor and Ivacaftor Exposure by Age Group Age Group Weight Dose Mean Lumacaftor (SD)*
AUCss (µg∙h/mL)Mean Ivacaftor (SD)†
AUCss (µg∙h/mL)Patients aged 1 to <2 years 7 kg to <9 kg lumacaftor 75 mg/ivacaftor 94 mg every 12 hours. 234 7.98 9 kg to <14 kg lumacaftor 100 mg/ivacaftor 125 mg every 12 hours. 191 (40.6) 5.35 (1.61) ≥14 kg lumacaftor 150 mg/ivacaftor 188 mg every 12 hours. 116 5.82 Patients aged 2 through 5 years <14 kg lumacaftor 100 mg/ivacaftor 125 mg every 12 hours. 180 (45.5) 5.92 (4.61) ≥14 kg lumacaftor 150 mg/ivacaftor 188 mg every 12 hours. 217 (48.6) 5.90 (1.93) Patients aged 6 through 11 years - lumacaftor 200 mg/ivacaftor 250 mg every 12 hours. 203 (57.4) 5.26 (3.08) Patients aged 12 to <18 years - lumacaftor 400 mg/ivacaftor 250 mg every 12 hours. 241 (61.4) 3.90 (1.56) Male and Female Patients
The pharmacokinetics of ORKAMBI was evaluated using a population PK analyses of data from clinical studies of lumacaftor given in combination with ivacaftor. Results indicate no clinically relevant difference in pharmacokinetic parameters for lumacaftor and ivacaftor between males and females.
Patients with Renal Impairment
Pharmacokinetic studies have not been performed with ORKAMBI in patients with renal impairment [see Use in Specific Populations (8.7)].
Patients with Hepatic Impairment
Following multiple doses of lumacaftor/ivacaftor for 10 days, subjects with moderately impaired hepatic function (Child-Pugh Class B, score 7 to 9) had approximately 50% higher exposures (AUC0-12h) and approximately 30% higher Cmax for both lumacaftor and ivacaftor compared with healthy subjects matched for demographics.
Pharmacokinetic studies have not been conducted in patients with mild (Child-Pugh Class A, score 5 to 6) or severe hepatic impairment (Child-Pugh Class C, score 10 to 15) receiving ORKAMBI [see Dosage and Administration (2.2), Warnings and Precautions (5.1), Adverse Reactions (6), and Use in Specific Populations (8.6)].
Drug Interaction Studies
Drug interaction studies were performed with lumacaftor/ivacaftor and other drugs likely to be co-administered or drugs commonly used as probes for pharmacokinetic interaction studies [see Drug Interactions (7)].
Potential for Lumacaftor/Ivacaftor to Affect Other Drugs
Lumacaftor is a strong inducer of CYP3A. Co-administration of lumacaftor with ivacaftor, a sensitive CYP3A substrate, decreased ivacaftor exposure by 80%. Ivacaftor is a weak inhibitor of CYP3A when given as monotherapy. The net effect of lumacaftor/ivacaftor therapy is strong CYP3A induction [see Drug Interactions (7.3)].
Based on in vitro results which showed P-gp inhibition and PXR activation, lumacaftor has the potential to both inhibit and induce P-gp. A clinical study with ivacaftor monotherapy showed that ivacaftor is a weak inhibitor of P-gp. Therefore, concomitant use of ORKAMBI with P-gp substrates may alter the exposure of these substrates [see Drug Interactions (7.5)].
In vitro studies suggest that lumacaftor has the potential to induce CYP2B6, CYP2C8, CYP2C9, and CYP2C19; inhibition of CYP2C8 and CYP2C9 has also been observed in vitro. In vitro studies suggest that ivacaftor may inhibit CYP2C9. Therefore, concomitant use of ORKAMBI with CYP2B6, CYP2C8, CYP2C9, and CYP2C19 substrates may alter the exposure of these substrates [see Drug Interactions (7.4)].
Potential for Other Drugs to Affect Lumacaftor/Ivacaftor
Lumacaftor exposure is not affected by concomitant CYP3A inducers or inhibitors. Exposure of ivacaftor when given in combination with lumacaftor is reduced by concomitant CYP3A inducers and increased by concomitant CYP3A inhibitors [see Dosage and Administration (2.3), Warnings and Precautions (5.6), and Drug Interactions (7)].
The effects of co-administered drugs on the exposure of lumacaftor and ivacaftor are shown in Table 6 [see Dosage and Administration (2.3), Warnings and Precautions (5.6), and Drug Interactions (7)].
Table 6: Impact of Other Drugs on Lumacaftor 200 mg q12h/Ivacaftor 250 mg q12h Co-administered Drug Dose of
Co-administered DrugEffect on PK* Mean Ratio (90% CI) of Lumacaftor and Ivacaftor
No Effect=1.0AUC Cmax CI = Confidence Interval; PK = Pharmacokinetics. CYP3A inhibitor: itraconazole 200 mg once daily ↔ Lumacaftor 0.97
(0.91, 1.02)0.99
(0.92, 1.05)↑ Ivacaftor 4.30†
(3.78, 4.88)3.64†
(3.19, 4.17)CYP3A inducer: rifampin 600 mg once daily ↔ Lumacaftor 0.87
(0.81, 0.93)0.96
(0.87, 1.05)↓ Ivacaftor 0.43
(0.38, 0.49)0.50
(0.43, 0.58)Other: ciprofloxacin 750 mg q12h ↔ Lumacaftor 0.86
(0.79, 0.95)0.88
(0.80, 0.97)↔ Ivacaftor 1.29
(1.12, 1.48)1.29
(1.11, 1.49) -
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with ORKAMBI; however, studies are available for ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with ORKAMBI; however, studies are available for individual components, lumacaftor and ivacaftor, as described below.
Lumacaftor
A two-year study in Sprague-Dawley rats and a 26-week study in transgenic Tg.rasH2 mice were conducted to assess carcinogenic potential of lumacaftor. No evidence of tumorigenicity was observed in rats at lumacaftor oral doses up to 1000 mg/kg/day (approximately 5 and 13 times the MRHD on a lumacaftor AUC basis in males and females, respectively). No evidence of tumorigenicity was observed in Tg.rasH2 mice at lumacaftor oral doses up to 1500 and 2000 mg/kg/day in female and male mice, respectively. Lumacaftor was negative for genotoxicity in the following assays: Ames test for bacterial gene mutation, in vitro chromosomal aberration assay in Chinese hamster ovary cells, and in vivo mouse micronucleus test.
Lumacaftor had no effects on fertility and reproductive performance indices in male and female rats at an oral dose of 1000 mg/kg/day (approximately 3 and 8 times, respectively, the MRHD on a lumacaftor AUC basis).
Ivacaftor
Two-year studies were conducted in mice and rats to assess carcinogenic potential of ivacaftor. No evidence of tumorigenicity was observed in mice and rats at ivacaftor oral doses up to 200 mg/kg/day and 50 mg/kg/day, respectively (approximately equivalent to 3 and 10 times the MRHD based on summed AUCs of ivacaftor and its metabolites).
Ivacaftor was negative for genotoxicity in the following assays: Ames test for bacterial gene mutation, in vitro chromosomal aberration assay in Chinese hamster ovary cells, and in vivo mouse micronucleus test.
Ivacaftor impaired fertility and reproductive performance indices in male and female rats at an oral dose of 200 mg/kg/day (approximately 15 and 7 times the MRHD based on summed AUCs of ivacaftor and its metabolites). Increases in prolonged diestrus were observed in females at 200 mg/kg/day. Ivacaftor also increased the number of females with all nonviable embryos and decreased corpora lutea, implantations, and viable embryos in rats at 200 mg/kg/day (approximately 7 times the MRHD based on summed AUCs of ivacaftor and its metabolites) when dams were dosed prior to and during early pregnancy. These impairments of fertility and reproductive performance in male and female rats at 200 mg/kg/day were attributed to severe toxicity. No effects on male or female fertility and reproductive performance indices were observed at an oral dose of ≤100 mg/kg/day (approximately 8 and 5 times the MRHD based on summed AUCs of ivacaftor and its metabolites).
-
14 CLINICAL STUDIESDose Ranging - Dose ranging for the clinical program consisted primarily of one double-blind, placebo-controlled, multiple-cohort trial which included 97 Caucasian patients with CF (homozygous ...
Dose Ranging
Dose ranging for the clinical program consisted primarily of one double-blind, placebo-controlled, multiple-cohort trial which included 97 Caucasian patients with CF (homozygous for the F508del mutation) aged 18 years and older with a screening ppFEV1 ≥40. In the trial, 76 patients (homozygous for the F508del mutation) were randomized to receive lumacaftor alone at once daily doses of 200 mg, 400 mg, or 600 mg or 400 mg q12h for 28 days followed by the addition of ivacaftor 250 mg q12h and 27 patients (homozygous or heterozygous for the F508del mutation) received placebo. During the initial 28-day lumacaftor monotherapy period, treatment with lumacaftor demonstrated a dose-dependent decrease in ppFEV1 compared to placebo. Changes from Day 1 at Day 28 in ppFEV1 compared to placebo were 0.24, -1.4, -2.7, and -4.6 for the 200 mg once daily, 400 mg once daily, 600 mg once daily, and 400 mg q12h lumacaftor doses, respectively. Following the addition of ivacaftor 250 mg q12h, the changes from Day 1 at Day 56 in ppFEV1 compared to placebo were 3.8, 2.7, 5.6, and 4.2, respectively.
Sweat chloride was also assessed in this trial. Following the initial 28 days of lumacaftor monotherapy, the changes from Day 1 at Day 28 in sweat chloride compared to placebo were -4.9, -8.3, -6.1, and -8.2 mmol/L for the 200 mg once daily, 400 mg once daily, 600 mg once daily, and 400 mg q12h lumacaftor doses, respectively. Following the addition of ivacaftor 250 mg q12h, the changes from Day 1 at Day 56 in sweat chloride compared to placebo were -5.0, -9.8, -9.5, and -11 mmol/L, respectively.
These data supported the evaluation of lumacaftor 400 mg/ivacaftor 250 mg q12h (ORKAMBI) and lumacaftor 600 mg once daily/ivacaftor 250 mg q12h in the confirmatory trials.
CloseConfirmatory
The efficacy of ORKAMBI in patients with CF who are homozygous for the F508del mutation in the CFTR gene was evaluated in two randomized, double-blind, placebo-controlled, 24-week clinical trials (Trials 1 and 2) in 1108 clinically stable patients with CF of whom 369 patients received ORKAMBI twice daily.
Trial 1 evaluated 549 patients with CF who were aged 12 years and older (mean age 25.1 years) with ppFEV1 at screening between 40-90 [mean ppFEV1 60.7 at baseline (range: 31.1 to 94.0)]. Trial 2 evaluated 559 patients aged 12 years and older (mean age 25.0 years) with ppFEV1 at screening between 40-90 [mean ppFEV1 60.5 at baseline (range: 31.3 to 99.8)]. Patients with a history of colonization with organisms such as Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus, or who had 3 or more abnormal liver function tests (ALT, AST, AP, GGT ≥3 × the ULN or total bilirubin ≥2 × the ULN) were excluded.
Patients in both trials were randomized 1:1:1 to receive either ORKAMBI (lumacaftor 400 mg q12h/ivacaftor 250 mg q12h; or lumacaftor 600 mg once daily/ivacaftor 250 mg q12h) or placebo. Patients took the study drug with fat-containing food for 24 weeks in addition to their prescribed CF therapies (e.g., bronchodilators, inhaled antibiotics, dornase alfa, and hypertonic saline).
The primary efficacy endpoint in both trials was change in lung function as determined by absolute change from baseline in ppFEV1 at Week 24, assessed as the average of the treatment effects at Week 16 and at Week 24. In both trials, treatment with ORKAMBI resulted in a statistically significant improvement in ppFEV1. The treatment difference between ORKAMBI and placebo for the mean absolute change in ppFEV1 from baseline at Week 24 (assessed as the average of the treatment effects at Week 16 and at Week 24) was 2.6 percentage points [95% CI (1.2, 4.0)] in Trial 1 (P=0.0003) and 3.0 percentage points [95% CI (1.6, 4.4)] in Trial 2 (P<0.0001). These changes persisted throughout the 24-week treatment period (see Figure 1). Improvements in ppFEV1 were observed regardless of age, disease severity, sex, and geographic region.
Figure 1. Absolute Change From Baseline at Each Visit in Percent Predicted FEV1 in Trial 1 and Trial 2. 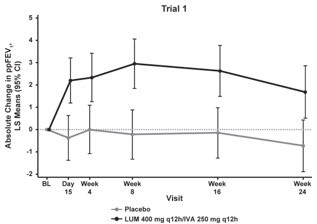
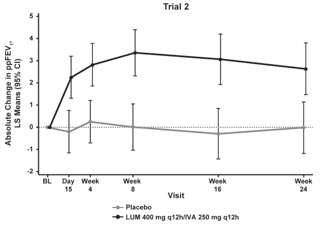
LS = Least Squares; q12h = every 12 hours Key secondary efficacy variables included relative change from baseline in ppFEV1 at Week 24, assessed as the average of the treatment effects at Week 16 and at Week 24; absolute change from baseline in BMI at Week 24; absolute change from baseline in Cystic Fibrosis Questionnaire-Revised (CFQ-R) Respiratory Domain score at Week 24, a measure of respiratory symptoms relevant to patients with CF such as cough, sputum production, and difficulty breathing; proportion of patients achieving ≥5% relative change from baseline in ppFEV1 using the average of Week 16 and Week 24; and number of pulmonary exacerbations through Week 24. For the purposes of these trials, a pulmonary exacerbation was defined as a change in antibiotic therapy (IV, inhaled, or oral) as a result of 4 or more of 12 pre-specified sino-pulmonary signs/symptoms.
Table 7: Summary of Other Efficacy Endpoints in Trials 1 and 2* Trial 1 Trial 2 Placebo
(n=184)ORKAMBI
LUM 400 mg q12h/IVA 250 mg q12h
(n=182)Placebo
(n=187)ORKAMBI
LUM 400 mg q12h/IVA 250 mg q12h
(n=187)- *
- In each trial, a hierarchical testing procedure was performed within each active treatment arm for primary and secondary endpoints vs. placebo; at each step, P≤0.0250 and all previous tests also meeting this level of significance was required for statistical significance.
- †
- Assessed as the average of the treatment effects at Week 16 and Week 24.
- ‡
- Indicates statistical significance confirmed in the hierarchical testing procedure. Other efficacy measures considered not statistically significant.
Relative change in ppFEV1 at Week 24† (%) Treatment difference
(95% CI)– 4.3
(1.9, 6.8)
P=0.0006‡– 5.3
(2.7, 7.8)
P<0.0001‡Absolute change in BMI at Week 24 (kg/m2) Treatment difference
(95% CI)– 0.1
(-0.1, 0.3)– 0.4
(0.2, 0.5)
P=0.0001‡Absolute change in CFQ-R Respiratory Domain Score (Points) at Week 24 Treatment difference
(95% CI)– 1.5
(-1.7, 4.7)– 2.9
(-0.3, 6.0)Proportion of patients with ≥5% relative change in ppFEV1 at Week 24† % 22% 37% 23% 41% Odds ratio
(95% CI)– 2.1
(1.3, 3.3)– 2.4
(1.5, 3.7)Number of pulmonary exacerbations through Week 24 # of events (rate per 48 weeks) 112 (1.1) 73 (0.7) 139 (1.2) 79 (0.7) Rate ratio
(95% CI)– 0.7
(0.5, 0.9)– 0.6
(0.4, 0.8) -
16 HOW SUPPLIED/STORAGE AND HANDLINGORKAMBI (lumacaftor 200 mg/ivacaftor 125 mg) is supplied as pink, oval-shaped tablets; each tablet contains 200 mg of lumacaftor and 125 mg of ivacaftor, printed with "2V125" in black ink on one ...
ORKAMBI (lumacaftor 200 mg/ivacaftor 125 mg) is supplied as pink, oval-shaped tablets; each tablet contains 200 mg of lumacaftor and 125 mg of ivacaftor, printed with "2V125" in black ink on one side and plain on the other, and is packaged as follows:
112–count tablet box containing a 4-week supply (4 weekly cartons of 7 daily blister strips with 4 tablets per strip). NDC 51167-809-01 ORKAMBI (lumacaftor 100 mg/ivacaftor 125 mg) is supplied as pink, oval-shaped tablets; each tablet contains 100 mg of lumacaftor and 125 mg of ivacaftor, printed with "1V125" in black ink on one side and plain on the other, and is packaged as follows:
112–count tablet box containing a 4-week supply (4 weekly cartons of 7 daily blister strips with 4 tablets per strip). NDC 51167-700-02 ORKAMBI (lumacaftor/ivacaftor) oral granules are supplied as small white to off-white granules and enclosed in unit-dose packets as follows:
56-count carton (contains 56 unit-dose packets of lumacaftor 75 mg/ivacaftor 94 mg per packet) NDC 51167-122-01 56-count carton (contains 56 unit-dose packets of lumacaftor 100 mg/ivacaftor 125 mg per packet) NDC 51167-900-01 56-count carton (contains 56 unit-dose packets of lumacaftor 150 mg/ivacaftor 188 mg per packet) NDC 51167-500-02 CloseStore at 20°C - 25°C (68°F - 77°F); excursions permitted to 15°C - 30°C (59°F - 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Advanced Liver Disease - Inform patients that worsening of liver function, including hepatic encephalopathy ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advanced Liver Disease
Inform patients that worsening of liver function, including hepatic encephalopathy, in patients with advanced liver disease occurred in some patients treated with ORKAMBI. Liver function decompensation, including liver failure leading to death, has been reported in CF patients with pre-existing cirrhosis with portal hypertension while receiving ORKAMBI [see Warnings and Precautions (5.1)].
Abnormalities in Liver Function and Testing
Inform patients that abnormalities in liver function have occurred in patients treated with ORKAMBI. Blood tests to measure transaminases (ALT and AST) and bilirubin will be performed prior to initiating ORKAMBI, every 3 months during the first year of therapy, and annually thereafter. Advise patients to contact their healthcare provider if they develop symptoms consistent with hepatotoxicity [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions including angioedema and anaphylaxis are possible with use of ORKAMBI. Inform patients of the early signs of hypersensitivity reactions including rash, hives, itching, facial swelling, tightness of the chest and wheezing. Advise patients to discontinue use of ORKAMBI immediately and contact their physician or go to the emergency department if these symptoms occur.
Respiratory Events
Inform patients that chest discomfort, dyspnea, and respiration abnormal were more common during initiation of ORKAMBI therapy, especially in patients with advanced lung disease and to contact their healthcare provider if they develop any of these symptoms [see Warnings and Precautions (5.4)].
Effect on Blood Pressure
Inform patients that increased blood pressure has been observed in some patients treated with ORKAMBI and that periodic monitoring of their blood pressure during treatment is recommended and to contact their healthcare provider if they develop elevated blood pressure or notice elevations in pre-existing high blood pressure [see Warnings and Precautions (5.5)].
Drug Interactions with CYP3A Inhibitors and Inducers
- Advise patients to inform their healthcare providers of all concomitant medications, herbal and dietary supplements. Advise patients to avoid grapefruit products during the first week after treatment initiation with ORKAMBI [see Dosage and Administration (2.3), Warnings and Precautions (5.6), and Drug Interactions (7)].
- Instruct post-menarchal patients including females of reproductive potential on alternative methods of birth control because hormonal contraceptives should not be relied upon as an effective method of contraception. ORKAMBI may decrease the effectiveness of hormonal contraceptives and there is an increased incidence of menstruation-related adverse reactions when co-administered with ORKAMBI [see Warnings and Precautions (5.6), Adverse Reactions (6.1), and Drug Interactions (7.11)].
Cataracts
Inform patients that abnormalities of the eye lens (cataract) have been noted in some children and adolescents receiving ORKAMBI. Advise pediatric patients and their caregivers that they will receive ophthalmological examinations before initiating and during ORKAMBI treatment. Advise pediatric patients and/or their caregivers to contact their healthcare provider if they experience visual changes [see Warnings and Precautions (5.7)].
CloseAdministration
Inform patients that ORKAMBI should be taken with fat-containing food. A typical CF diet will satisfy this requirement. Examples of fat-containing foods include eggs, avocados, nuts, butter, peanut butter, cheese pizza, breast milk, infant formula, whole-milk dairy products (such as whole milk, cheese, and yogurt), etc. [see Dosage and Administration (2.1) and Clinical Pharmacology (12.3)].
Inform patients and caregivers that ORKAMBI oral granules should be mixed with one teaspoon (5 mL) of age-appropriate soft food or liquid and completely consumed to ensure delivery of the entire dose. Food or liquid should be at or below room temperature. Once mixed, the product has been shown to be stable for one hour, and therefore should be consumed during this period. Some examples of appropriate soft foods or liquids may include puréed fruits or vegetables, flavored yogurt or pudding, applesauce, water, milk, breast milk, infant formula or juice.
Inform patients that if a dose is missed and they remember the missed dose within 6 hours, the patients should take the dose with fat-containing food. If more than 6 hours elapsed after the usual dosing time, the patients should skip that dose and resume the normal schedule for the following dose. Patients should be informed not to take a double dose to make up for the forgotten dose [see Dosage and Administration (2.1)].
-
SPL UNCLASSIFIED SECTIONManufactured for - Vertex Pharmaceuticals Incorporated - 50 Northern Avenue - Boston, MA 02210 - ORKAMBI, the ORKAMBI logo, VERTEX, and the VERTEX triangle logo are registered trademarks of Vertex ...
Manufactured for
Vertex Pharmaceuticals Incorporated
50 Northern Avenue
Boston, MA 02210ORKAMBI, the ORKAMBI logo, VERTEX, and the VERTEX triangle logo are registered trademarks of Vertex Pharmaceuticals Incorporated.
All other trademarks referenced herein are the property of their respective owners.
©2024 Vertex Pharmaceuticals Incorporated
ALL RIGHTS RESERVED
Close -
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 08/2023 - PATIENT INFORMATION - ORKAMBI (or-KAM-bee) (lumacaftor and ivacaftor) tablets for ...Close
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 08/2023 PATIENT INFORMATION
ORKAMBI (or-KAM-bee)
(lumacaftor and ivacaftor) tablets for oral use
(lumacaftor and ivacaftor) oral granulesWhat is ORKAMBI?
- ORKAMBI is a prescription medicine used for the treatment of cystic fibrosis (CF) in people aged 1 year and older who have two copies of the F508del mutation (F508del/F508del) in their CFTR gene.
- ORKAMBI should not be used in people other than those who have two copies of the F508del mutation in their CFTR gene.
It is not known if ORKAMBI is safe and effective in children under 1 year of age.
Before taking ORKAMBI, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems.
- are allergic to ORKAMBI or any ingredients in ORKAMBI. See the end of this patient information leaflet for a complete list of ingredients in ORKAMBI.
- have kidney problems.
- have lung problems.
- have had an organ transplant.
- are using birth control (hormonal contraceptives, including oral, injectable, transdermal, or implantable forms). Hormonal contraceptives should not be used as a method of birth control when taking ORKAMBI. Talk to your doctor about the best birth control method you should use while taking ORKAMBI.
- are pregnant or plan to become pregnant. It is not known if ORKAMBI will harm your unborn baby. You and your doctor should decide if you will take ORKAMBI while you are pregnant.
- are breastfeeding or planning to breastfeed. It is not known if ORKAMBI passes into your breast milk. You and your doctor should decide if you will take ORKAMBI while you are breastfeeding.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
ORKAMBI may affect the way other medicines work, and other medicines may affect how ORKAMBI works. The dose of ORKAMBI may need to be adjusted when taken with certain medicines. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Especially tell your doctor if you take:
- antibiotics: rifampin (RIFAMATE®, RIFATER®) or rifabutin (MYCOBUTIN®).
- seizure medicines: phenobarbital, carbamazepine (TEGRETOL®, CARBATROL®, EQUETRO®), or phenytoin (DILANTIN®, PHENYTEK®).
- sedatives and anti-anxiety medicines: triazolam (HALCION®) or midazolam (DORMICUM®, HYPNOVEL®, and VERSED®).
- immunosuppressant medicines: cyclosporine, everolimus (ZORTRESS®), sirolimus (RAPAMUNE®), or tacrolimus (ASTAGRAF XL®, ENVARSUS® XR, PROGRAF®, and PROTOPIC®).
- St. John's wort (Hypericum perforatum).
- antifungal medicines including ketoconazole, itraconazole (such as SPORANOX®), posaconazole (such as NOXAFIL®), or voriconazole (such as VFEND®).
- antibiotics including telithromycin, clarithromycin (such as BIAXIN®), or erythromycin (such as ERY-TAB®).
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take ORKAMBI?
- Take ORKAMBI exactly as your doctor tells you to take it.
- Always take ORKAMBI tablets or granules with foods that contain fat. Examples of fat-containing foods include eggs, avocados, nuts, butter, peanut butter, cheese pizza, breast milk, infant formula, whole-milk dairy products (such as whole milk, cheese, and yogurt).
- Take your doses of ORKAMBI 12 hours apart.
- ORKAMBI tablets (aged 6 years and older):
- Each ORKAMBI box contains 4 weekly cartons.
- Each carton contains 7 daily blister strips.
- Each blister strip contains 4 tablets so you can take 2 tablets for the morning and 2 tablets for the evening.
- You may cut along the dotted line to separate your morning dose from your evening dose.
- To take your morning dose, unpeel the paper backing from a blister strip (do not push tablet through backing) to remove 2 ORKAMBI tablets and take them with fat-containing food.
- 12 hours after your previous dose, open another blister strip (do not push tablet through backing) to remove 2 ORKAMBI tablets and take them with fat-containing food.
- ORKAMBI oral granules (aged 1 to under 6 years old):
- Hold the packet with the cut line on top.
- Shake the packet gently to settle the ORKAMBI granules.
- Tear or cut packet open along cut line.
- Carefully pour all of the ORKAMBI granules in the packet into one teaspoon (5 mL) of soft food or liquid in a small container (like an empty bowl).
- The food or liquid should be at or below room temperature. Examples of soft foods or liquids include puréed fruits or vegetables, flavored yogurt or pudding, applesauce, water, milk, breast milk, infant formula or juice.Mix the ORKAMBI granules with food or liquid.
- After mixing, give ORKAMBI within 1 hour. Make sure all medicine is taken.
- Give a child fat-containing food just before or just after the ORKAMBI granules dose (see examples above).
- If you miss a dose within 6 hours of when you usually take it, take your dose with fat-containing food as soon as possible.
- If you miss a dose and it is more than 6 hours after the time you usually take it, skip that dose only and take the next dose when you usually take it. Do not take 2 doses at the same time to make up for your missed dose.
- Tell your doctor if you stop ORKAMBI for more than 1-week. Your doctor may need to change your dose of ORKAMBI or other medicines you take.
What should I avoid while taking ORKAMBI?
Do not eat or drink grapefruit products during your first week of treatment with ORKAMBI. Eating or drinking grapefruit products can increase the amount of ORKAMBI in your blood.What are the possible side effects of ORKAMBI?
ORKAMBI can cause serious side effects, including:
-
Worsening of liver function in people with severe liver disease. The worsening of liver function can be serious or cause death. Talk to your doctor if you have been told you have liver disease as your doctor may need to adjust the dose of ORKAMBI.
-
High liver enzymes in the blood, which can be a sign of liver injury in people receiving ORKAMBI. Your doctor will do blood tests to check your liver:
- before you start ORKAMBI
- every 3 months during your first year of taking ORKAMBI
- every year while you are taking ORKAMBI
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- loss of appetite
- dark, amber-colored urine
- yellowing of your skin or the white part of your eyes
- nausea or vomiting
- confusion
- Serious Allergic Reactions can happen to people who are treated with ORKAMBI. Call your doctor or go to the emergency room right away if you have any symptoms of an allergic reaction. Symptoms of an allergic reaction may include:
- rash or hives
- tightness of the chest or throat or difficulty breathing
- swelling of the face, lips, and/or tongue, difficulty swallowing
- light-headedness or dizziness
- Breathing problems such as trouble breathing, shortness of breath or chest tightness in people when starting ORKAMBI, especially in people who have poor lung function. If you have poor lung function, your doctor may monitor you more closely when you start ORKAMBI. Call your doctor right away if you have trouble breathing, shortness of breath or chest tightness.
- An increase in blood pressure in some people receiving ORKAMBI. Your doctor should monitor your blood pressure during treatment with ORKAMBI. Call your doctor right away if you have an increase in blood pressure.
- Abnormality of the eye lens (cataract) in some children and adolescents receiving ORKAMBI. If you are a child or adolescent, your doctor should perform eye examinations before and during treatment with ORKAMBI to look for cataracts.
- breathing problems such as shortness of breath and chest tightness
- nausea
- diarrhea
- fatigue
- increase in a certain blood enzyme called creatine phosphokinase
- rash
- gas
- common cold, including sore throat, stuffy or runny nose
- flu or flu-like symptoms
- irregular, missed, or abnormal periods (menses) and increase in the amount of menstrual bleeding
Additional side effects in children
Side effects seen in children are similar to those seen in adults and adolescents. Additional common side effects seen in children include:
- cough with sputum
- stuffy nose
- headache
- stomach pain
- increase in sputum
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ORKAMBI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ORKAMBI?
- Store ORKAMBI at room temperature between 68°F to 77°F (20°C to 25°C).
Keep ORKAMBI and all medicines out of the reach of children.
General information about the safe and effective use of ORKAMBI.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ORKAMBI for a condition for which it was not prescribed. Do not give ORKAMBI to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or doctor for information about ORKAMBI that is written for health professionals.
What are the ingredients in ORKAMBI?
ORKAMBI tablets:
Active ingredients: lumacaftor and ivacaftor
Inactive ingredients: cellulose, microcrystalline; croscarmellose sodium; hypromellose acetate succinate; magnesium stearate; povidone; and sodium lauryl sulfate.The tablet film coat contains: carmine, FD&C Blue #1, FD&C Blue #2, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
The printing ink contains: ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
ORKAMBI oral granules
Active ingredients: lumacaftor and ivacaftor
Inactive ingredients: cellulose, microcrystalline; croscarmellose sodium; hypromellose acetate succinate; povidone; and sodium lauryl sulfate.Manufactured for: Vertex Pharmaceuticals Incorporated; 50 Northern Avenue, Boston, MA 02210
For more information, go to www.orkambi.com or call 1-877-752-5933.
ORKAMBI, the ORKAMBI logo, VERTEX, and the VERTEX triangle logo are registered trademarks of Vertex Pharmaceuticals Incorporated.
All other trademarks referenced herein are the property of their respective owners.
©2023 Vertex Pharmaceuticals Incorporated -
PRINCIPAL DISPLAY PANEL - 200 mg/125 mg tablet BoxRx only - NDC 51167-809-01 - ORKAMBI® (Lumacaftor/Ivacaftor) tablets - 200 mg/125 mg per tablet - 112 tablets
Rx only
NDC 51167-809-01ORKAMBI®
(Lumacaftor/Ivacaftor) tablets
200 mg/125 mg per tablet112 tablets
Close
-
PRINCIPAL DISPLAY PANEL - 100 mg/125 mg tablet BoxRx only - NDC 51167-700-02 - ORKAMBI® (Lumacaftor/Ivacaftor) tablets - 100 mg/125 mg per tablet - 112 tablets
Rx only
NDC 51167-700-02ORKAMBI®
(Lumacaftor/Ivacaftor) tablets
100 mg/125 mg per tablet112 tablets
Close
-
PRINCIPAL DISPLAY PANEL - 100 mg/125 mg packet CartonRx only - NDC 51167-900-01 - ORKAMBI® (Lumacaftor/Ivacaftor) 100 mg - 125 mg - Oral Granules - 100 mg/125 mg per packet - 56 packets - Carton contains: 4 individual wallets - with 14 packets per wallet - Lift ...
Rx only
NDC 51167-900-01ORKAMBI®
(Lumacaftor/Ivacaftor)100 mg
125 mgOral Granules
100 mg/125 mg per packet56 packets
Carton contains: 4 individual wallets
with 14 packets per walletLift here to open
Close
-
PRINCIPAL DISPLAY PANEL - 150 mg/188 mg packet CartonRx only - NDC 51167-500-02 - ORKAMBI® (Lumacaftor/Ivacaftor) 150 mg - 188 mg - Oral Granules - 150 mg/188 mg per packet - 56 packets - Carton contains: 4 individual wallets - with 14 packets per wallet - Lift ...
Rx only
NDC 51167-500-02
ORKAMBI®
(Lumacaftor/Ivacaftor)150 mg
188 mgOral Granules
150 mg/188 mg per packet56 packets
Carton contains: 4 individual wallets
with 14 packets per walletLift here to open
Close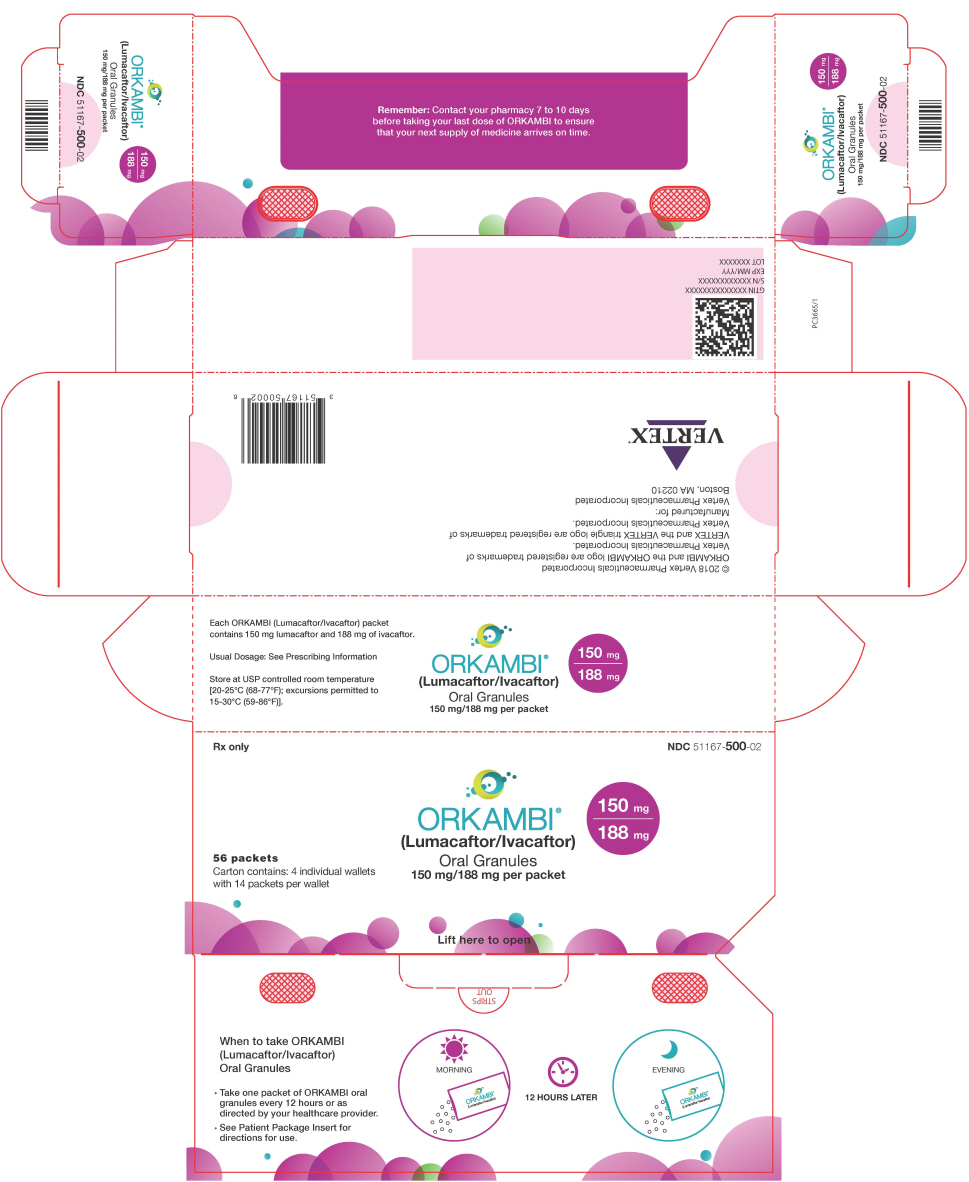
-
PRINCIPAL DISPLAY PANEL - 75 mg/94 mg packet CartonRx only - NDC 51167-122-01 - ORKAMBI® (Lumacaftor/Ivacaftor) 75 mg - 94 mg - Oral Granules - 75 mg/94 mg per packet - 56 packets - Carton contains: 4 individual wallets - with 14 packets per wallet - Lift here ...
Rx only
NDC 51167-122-01
ORKAMBI®
(Lumacaftor/Ivacaftor)75 mg
94 mgOral Granules
75 mg/94 mg per packet56 packets
Carton contains: 4 individual wallets
with 14 packets per walletLift here to open
Close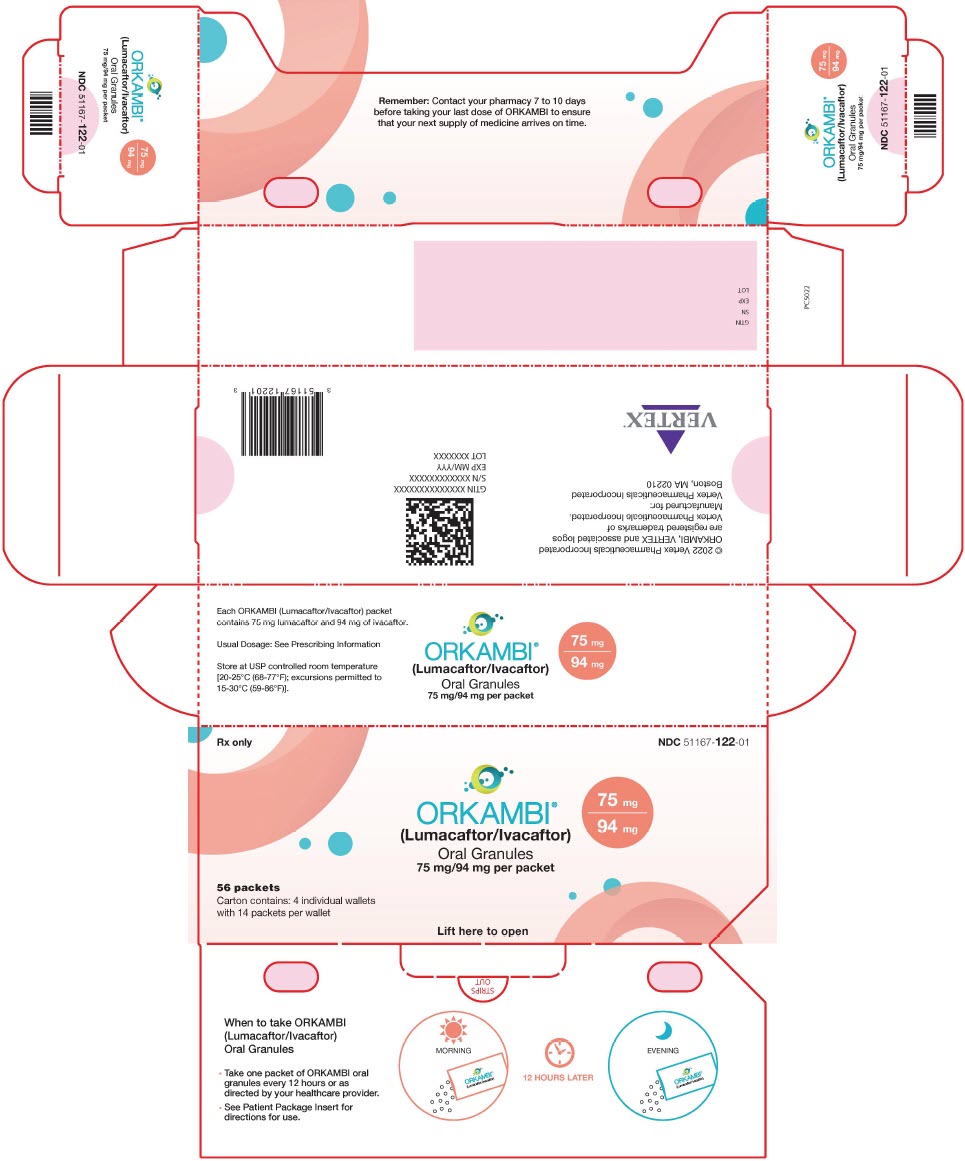
-
INGREDIENTS AND APPEARANCEProduct Information
ORKAMBI lumacaftor and ivacaftor tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51167-809 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lumacaftor (UNII: EGP8L81APK) (lumacaftor - UNII:EGP8L81APK) lumacaftor 200 mg ivacaftor (UNII: 1Y740ILL1Z) (ivacaftor - UNII:1Y740ILL1Z) ivacaftor 125 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE ACETATE SUCCINATE 06081224 (3 MPA.S) (UNII: 6N003M473W) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARMINIC ACID (UNII: CID8Z8N95N) FD&C Blue No. 1 (UNII: H3R47K3TBD) FD&C Blue No. 2 (UNII: L06K8R7DQK) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color PINK Score no score Shape OVAL Size 14mm Flavor Imprint Code 2V125 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51167-809-01 4 in 1 BOX 07/02/2015 1 7 in 1 CARTON 1 4 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206038 07/02/2015 ORKAMBI lumacaftor and ivacaftor tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51167-700 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lumacaftor (UNII: EGP8L81APK) (lumacaftor - UNII:EGP8L81APK) lumacaftor 100 mg ivacaftor (UNII: 1Y740ILL1Z) (ivacaftor - UNII:1Y740ILL1Z) ivacaftor 125 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE ACETATE SUCCINATE 06081224 (3 MPA.S) (UNII: 6N003M473W) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARMINIC ACID (UNII: CID8Z8N95N) FD&C Blue No. 1 (UNII: H3R47K3TBD) FD&C Blue No. 2 (UNII: L06K8R7DQK) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color PINK Score no score Shape OVAL Size 14mm Flavor Imprint Code 1V125 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51167-700-02 4 in 1 BOX 09/28/2016 1 7 in 1 CARTON 1 4 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206038 09/28/2016 ORKAMBI lumacaftor and ivacaftor granule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51167-900 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lumacaftor (UNII: EGP8L81APK) (lumacaftor - UNII:EGP8L81APK) lumacaftor 100 mg ivacaftor (UNII: 1Y740ILL1Z) (ivacaftor - UNII:1Y740ILL1Z) ivacaftor 125 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE ACETATE SUCCINATE 06081224 (3 MPA.S) (UNII: 6N003M473W) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51167-900-01 56 in 1 CARTON 08/07/2018 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211358 08/07/2018 ORKAMBI lumacaftor and ivacaftor granule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51167-500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lumacaftor (UNII: EGP8L81APK) (lumacaftor - UNII:EGP8L81APK) lumacaftor 150 mg ivacaftor (UNII: 1Y740ILL1Z) (ivacaftor - UNII:1Y740ILL1Z) ivacaftor 188 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE ACETATE SUCCINATE 06081224 (3 MPA.S) (UNII: 6N003M473W) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51167-500-02 56 in 1 CARTON 08/07/2018 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211358 08/07/2018 ORKAMBI lumacaftor and ivacaftor granule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51167-122 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength lumacaftor (UNII: EGP8L81APK) (lumacaftor - UNII:EGP8L81APK) lumacaftor 75 mg ivacaftor (UNII: 1Y740ILL1Z) (ivacaftor - UNII:1Y740ILL1Z) ivacaftor 94 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE ACETATE SUCCINATE 06081224 (3 MPA.S) (UNII: 6N003M473W) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51167-122-01 56 in 1 CARTON 09/02/2022 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211358 09/02/2022
CloseLabeler - Vertex Pharmaceuticals Incorporated (602478257)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ORKAMBI- lumacaftor and ivacaftor tablet, film coated
ORKAMBI- lumacaftor and ivacaftor granule
Number of versions: 20
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Dec 23, 2024 | 21 (current) | download |
| Jun 28, 2024 | 20 | download |
| Aug 17, 2023 | 19 | download |
| Mar 13, 2023 | 17 | download |
| Sep 27, 2022 | 16 | download |
| Sep 12, 2022 | 15 | download |
| Aug 5, 2022 | 14 | download |
| May 20, 2020 | 13 | download |
| Jul 25, 2019 | 12 | download |
| Dec 17, 2018 | 11 | download |
| Aug 16, 2018 | 10 | download |
| May 11, 2018 | 9 | download |
| Jan 30, 2018 | 8 | download |
| Dec 20, 2017 | 7 | download |
| Jun 14, 2017 | 6 | download |
| Apr 14, 2017 | 5 | download |
| Oct 7, 2016 | 4 | download |
| Sep 16, 2016 | 3 | download |
| May 26, 2016 | 2 | download |
| Jul 7, 2015 | 1 | download |
RxNorm
ORKAMBI- lumacaftor and ivacaftor tablet, film coated
ORKAMBI- lumacaftor and ivacaftor granule
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1655928 | lumacaftor 200 MG / ivacaftor 125 MG Oral Tablet | PSN |
| 2 | 1655928 | ivacaftor 125 MG / lumacaftor 200 MG Oral Tablet | SCD |
| 3 | 1655934 | ORKAMBI 200 MG / 125 MG Oral Tablet | PSN |
| 4 | 1655934 | ivacaftor 125 MG / lumacaftor 200 MG Oral Tablet [ORKAMBI] | SBD |
| 5 | 1655934 | ORKAMBI (lumacaftor 200 MG / ivacaftor 125 MG) Oral Tablet | SY |
| 6 | 1812468 | lumacaftor 100 MG / ivacaftor 125 MG Oral Tablet | PSN |
| 7 | 1812468 | ivacaftor 125 MG / lumacaftor 100 MG Oral Tablet | SCD |
| 8 | 1812470 | ORKAMBI 100 MG / 125 MG Oral Tablet | PSN |
| 9 | 1812470 | ivacaftor 125 MG / lumacaftor 100 MG Oral Tablet [ORKAMBI] | SBD |
| 10 | 1812470 | ORKAMBI (lumacaftor 100 MG / ivacaftor 125 MG) Oral Tablet | SY |
| 11 | 2053509 | ivacaftor 125 MG / lumacaftor 100 MG Oral Granules | PSN |
| 12 | 2053509 | ivacaftor 125 MG / lumacaftor 100 MG Oral Granules | SCD |
| 13 | 2053512 | ORKAMBI 100 MG / 125 MG Oral Granules | PSN |
| 14 | 2053512 | ivacaftor 125 MG / lumacaftor 100 MG Oral Granules [ORKAMBI] | SBD |
| 15 | 2053512 | ORKAMBI (ivacaftor 125 MG / lumacaftor 100 MG) Oral Granules | SY |
| 16 | 2053517 | ivacaftor 188 MG / lumacaftor 150 MG Oral Granules | PSN |
| 17 | 2053517 | ivacaftor 188 MG / lumacaftor 150 MG Oral Granules | SCD |
| 18 | 2053519 | ORKAMBI 150 MG / 188 MG Oral Granules | PSN |
| 19 | 2053519 | ivacaftor 188 MG / lumacaftor 150 MG Oral Granules [ORKAMBI] | SBD |
| 20 | 2053519 | ORKAMBI (ivacaftor 188 MG / lumacaftor 150 MG) Oral Granules | SY |
| 21 | 2611458 | ivacaftor 94 MG / lumacaftor 75 MG Oral Granules | PSN |
| 22 | 2611458 | ivacaftor 94 MG / lumacaftor 75 MG Oral Granules | SCD |
| 23 | 2611460 | ORKAMBI 94 MG / 75 MG Oral Granules | PSN |
| 24 | 2611460 | ivacaftor 94 MG / lumacaftor 75 MG Oral Granules [ORKAMBI] | SBD |
| 25 | 2611460 | ORKAMBI (ivacaftor 94 MG / lumacaftor 75 MG) Oral Granules | SY |
Get Label RSS Feed for this Drug
ORKAMBI- lumacaftor and ivacaftor tablet, film coated
ORKAMBI- lumacaftor and ivacaftor granule
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=3fc1c40e-cfac-47a1-9e1a-61ead3570600
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ORKAMBI- lumacaftor and ivacaftor tablet, film coated
ORKAMBI- lumacaftor and ivacaftor granule
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 51167-122-01 |
| 2 | 51167-500-02 |
| 3 | 51167-700-02 |
| 4 | 51167-809-01 |
| 5 | 51167-900-01 |