Label: ORAVIG- miconazole tablet
- NDC Code(s): 61825-303-14
- Packager: Galt Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONHIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ORAVIG safely and effectively. See full prescribing information for ORAVIG. ORAVIG ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGEORAVIG is indicated for the local treatment of oropharyngeal candidiasis (OPC) in adults.
-
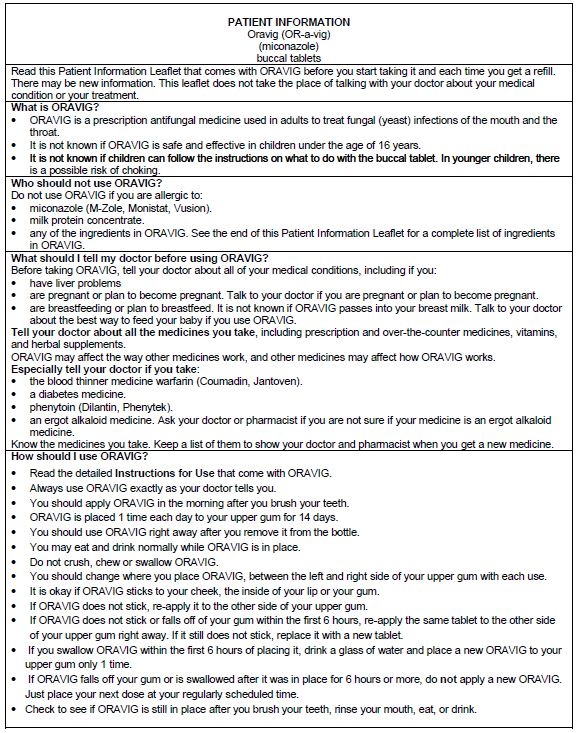
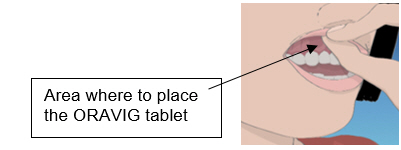
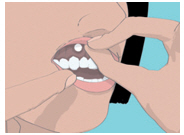
2 DOSAGE & ADMINISTRATION2.1 Basic Dosing Information - The recommended dosing schedule for ORAVIG is the application of one 50 mg buccal tablet to the upper gum region (canine fossa) once daily for 14 consecutive ...
-
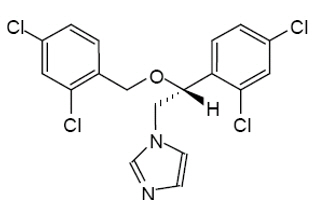
3 DOSAGE FORMS & STRENGTHSORAVIG is a buccal tablet containing 50 mg of miconazole. ORAVIG tablets are round, off-white tablets, with a rounded side and a flat side. The tablets are marked with an “L” on the flat ...
-
4 CONTRAINDICATIONSORAVIG is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to miconazole, milk protein concentrate, or any other component of the product.
-
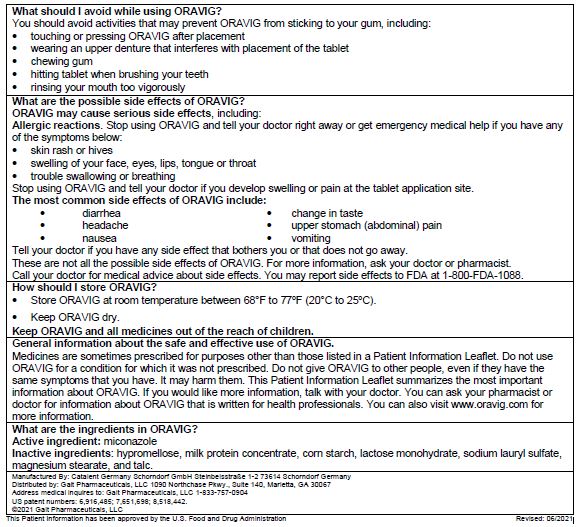
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Allergic reactions, including anaphylactic reactions and hypersensitivity, have been reported with the administration of miconazole products, including ORAVIG. Discontinue ...
-
6 ADVERSE REACTIONSThe following serious adverse drug reactions are discussed in detail in other sections of labeling: Hypersensitivity reactions - [see Warnings and Precautions (5.1)] 6.1 ...
-
7 DRUG INTERACTIONS7.1 Warfarin - Concomitant administration of miconazole and warfarin has resulted in enhancement of anticoagulant effect. Cases of bleeding and bruising following the concomitant use of warfarin ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal data, ORAVIG may cause fetal harm when administered to pregnant women. There are no available data on ORAVIG use in pregnant ...
-
10 OVERDOSAGEOverdose with miconazole in humans has not been reported in the literature. Miconazole absorption and systemic exposure following application of ORAVIG are minimal - [see Clinical ...
-
11 DESCRIPTIONORAVIG (miconazole) buccal tablets are applied topically to the gum once daily and release miconazole as the buccal tablet gradually dissolves - [see Clinical Pharmacology (12.3)] ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Miconazole is an antifungal drug - [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption and Distribution - Salivary - Single dose application ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Carcinogenicity studies with miconazole have not been conducted. Miconazole nitrate was not genotoxic when tested in ...
-
14 CLINICAL STUDIESStudy in HIV Infected Patients - The efficacy and safety of ORAVIG in the treatment of OPC was evaluated in a randomized, double-blind, double-dummy, multicenter trial comparing ORAVIG 50 mg once ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGORAVIG 50 mg buccal tablets are supplied as off-white tablets containing 50 mg of miconazole. ORAVIG tablets have a rounded side and a flat side. ORAVIG tablets are packaged in bottles of 14 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Important Administration Instructions - The tablet should be used ...
-
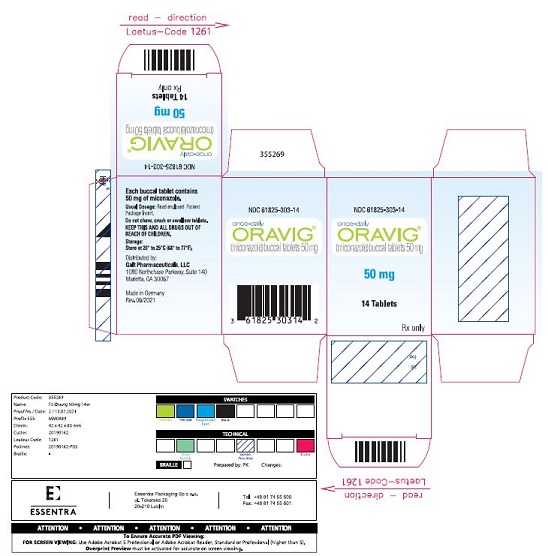
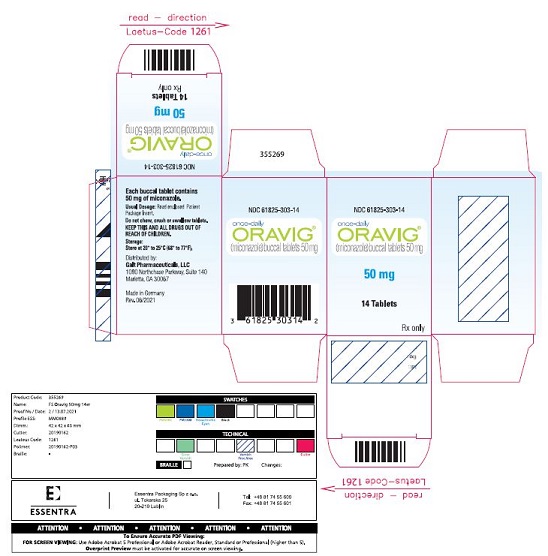
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 61825-303-14 - once-daily - ORAVIG® (miconazole) buccal tablets 50 mg - 50 mg - 14 Tablets - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information