Label: ORAPRED ODT- prednisolone sodium phosphate tablet, orally disintegrating
- NDC Code(s): 59212-700-06, 59212-700-12, 59212-700-48, 59212-701-02, view more
- Packager: Advanz Pharma (US) Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 25, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ORAPRED ODT® safely and effectively. See full prescribing information for ORAPRED ODT®. ORAPRED ODT® (prednisolone sodium ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEOrapred ODT (prednisolone sodium phosphate orally disintegrating tablet) is indicated in the treatment of the following diseases or conditions: 1.1 Allergic Conditions - Control of severe or ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - Dosage of Orapred ODT should be individualized according to the severity of the disease and the response of the patient. For pediatric patients, the recommended dosage ...
-
3 DOSAGE FORMS AND STRENGTHSOrally disintegrating tablets: 10 mg prednisolone (as 13.4 mg prednisolone sodium phosphate) 15 mg prednisolone (as 20.2 mg prednisolone sodium phosphate) 30 mg prednisolone (as 40.3 mg ...
-
4 CONTRAINDICATIONSOrapred ODT is contraindicated in patients who are hypersensitive to corticosteroids such as prednisolone or any components of this product. Rare instances of anaphylactoid reactions have occurred ...
-
5 WARNINGS AND PRECAUTIONS5.1 Alterations in Endocrine Function - Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome, and hyperglycemia. Monitor patients for these conditions with chronic ...
-
6 ADVERSE REACTIONSCommon adverse reactions for corticosteroids include fluid retention, alteration in glucose tolerance, elevation in blood pressure, behavioral and mood changes, increased appetite and weight ...
-
7 DRUG INTERACTIONSAminoglutethimide: Aminoglutethimide may lead to loss of corticosteroid-induced adrenal suppression. Amphotericin B: There have been cases reported in which concomitant use of Amphotericin B ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from human and animal studies, corticosteroids including Orapred, can cause fetal harm when administered to a pregnant woman (see Data) [see ...
-
10 OVERDOSAGEThe effects of accidental ingestion of large quantities of prednisolone over a very short period of time have not been reported, but prolonged use of the drug can produce mental symptoms, moon ...
-
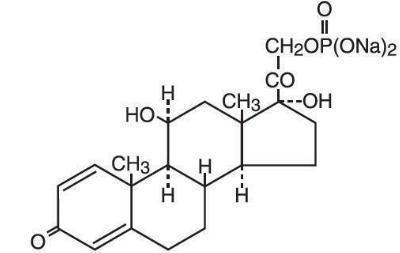
11 DESCRIPTIONOrapred ODT (prednisolone sodium phosphate disintegrating tablets) is a sodium salt of the phosphoester of the glucocorticoid prednisolone. Glucocorticoids are adrenocortical steroids, both ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Prednisolone is a synthetic adrenocortical steroid drug with predominantly glucocorticoid properties. Some of these properties reproduce the physiological actions of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Orapred was not formally evaluated in carcinogenicity studies. Review of the published literature identified the potential for ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGOrapred ODT (prednisolone sodium phosphate orally disintegrating tablets) 13.4 mg prednisolone sodium phosphate (equivalent to 10 mg prednisolone base) is a white, flat faced, beveled tablet ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients not to discontinue the use of Orapred abruptly or without medical supervision, to advise any healthcare provider that they are taking it, and to seek medical advice at once should ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Advanz Pharma (US) Corp. Bannockburn, IL 60015 - US Patent No. 6,740,341 - “ORAPRED® is a registered trademark of Mercury Pharma Group Limited. Distributed by Advanz Pharma ...
-
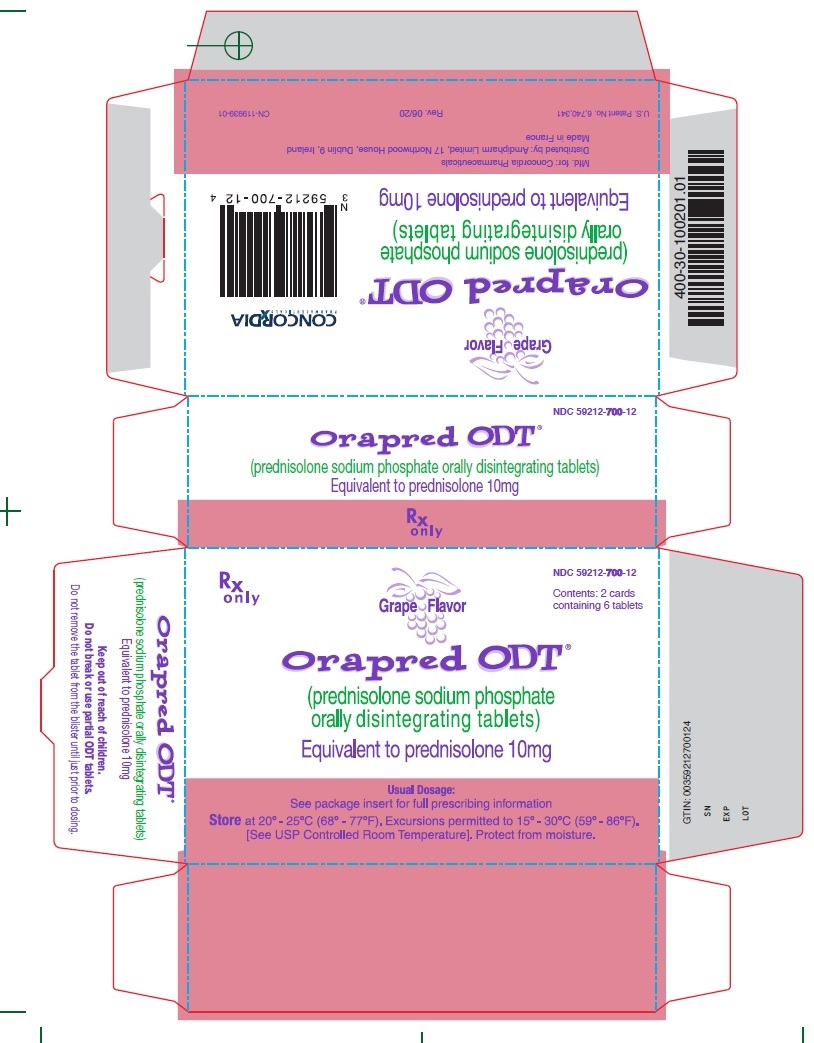
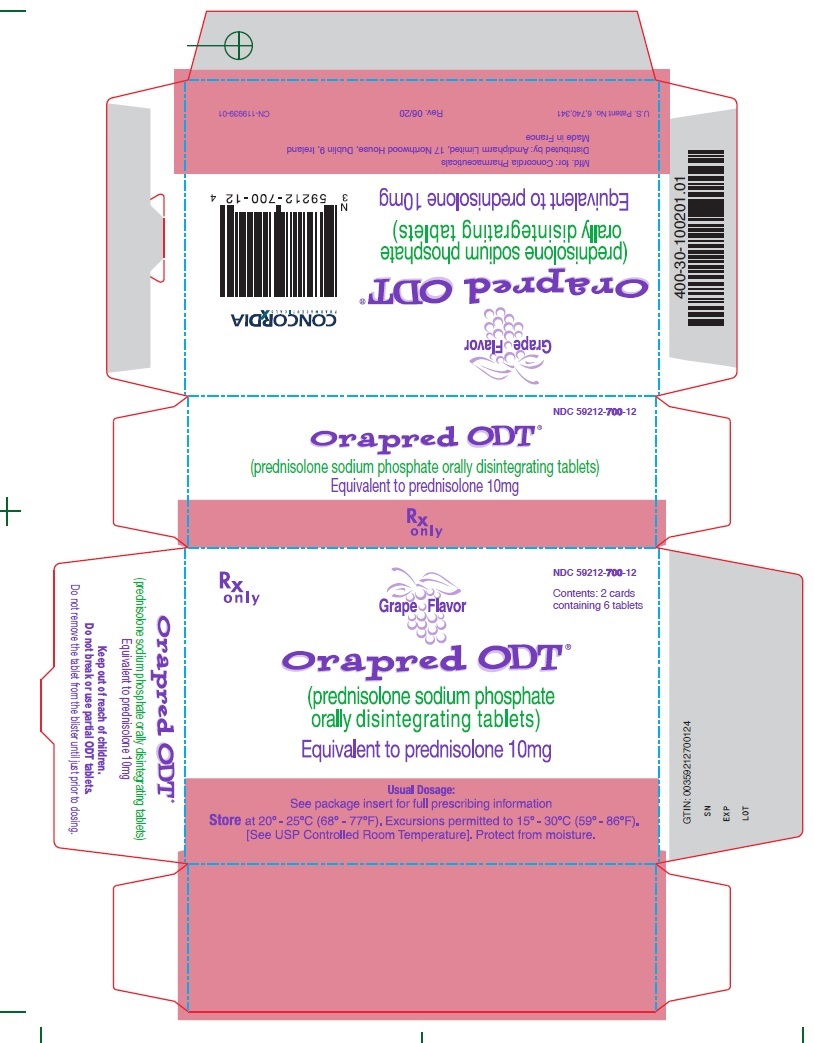
10 mg CartonRx only - NDC 59212-700-12 - Contents: 2 cards containing 6 tablets - Grape Flavor - Orapred ODT® (prednisolone sodium phosphate orally disintegrating tablets) Equivalent to prednisolone ...
-
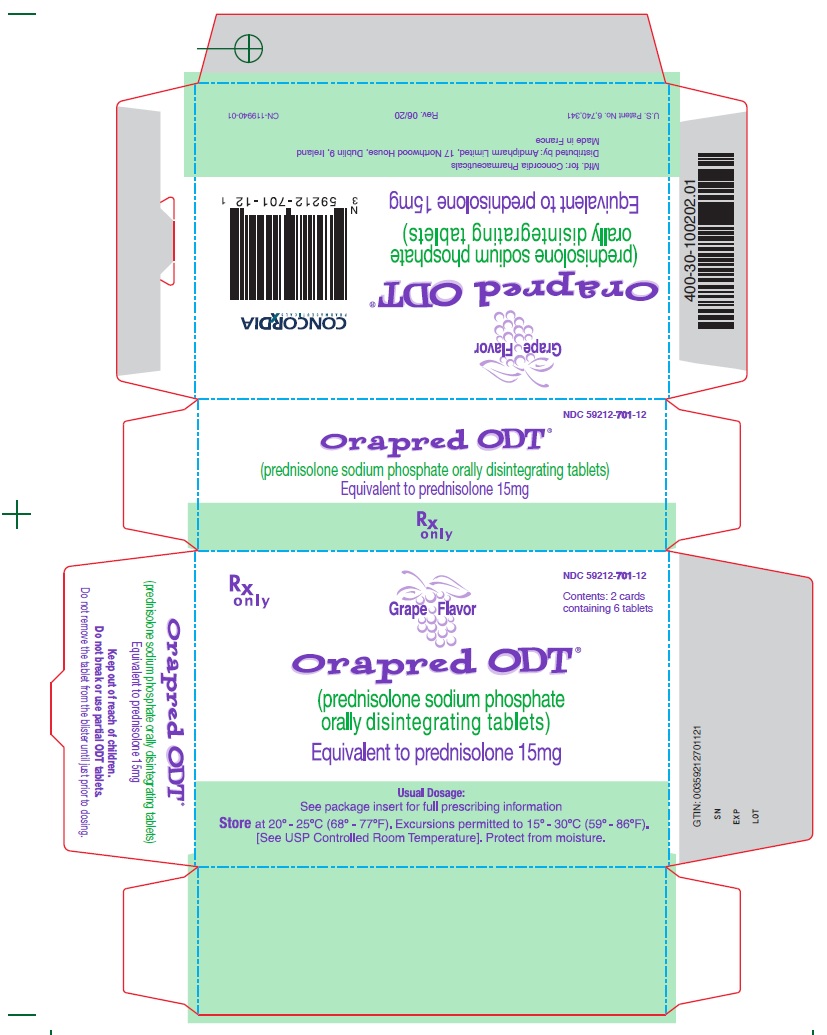
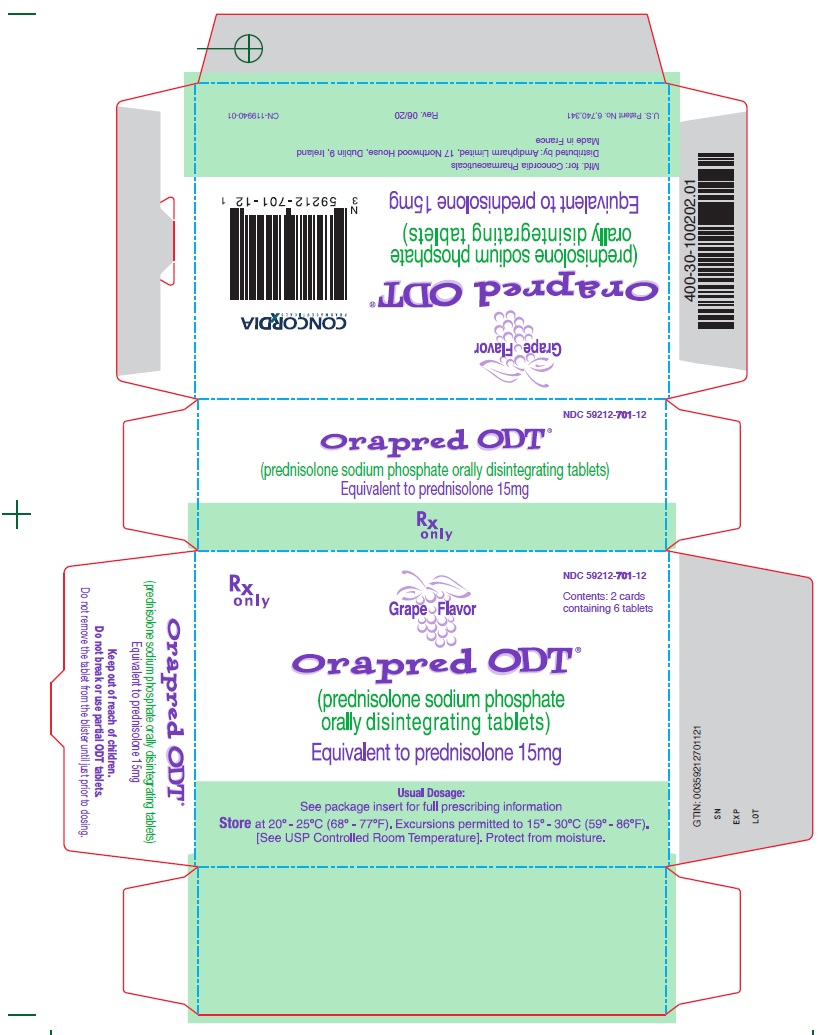
15 mg CartonRx only - NDC 59212-701-12 - Contents: 2 cards containing 6 tablets - Grape Flavor - Orapred ODT® (prednisolone sodium phosphate orally disintegrating tablets) Equivalent to prednisolone 15mg
-
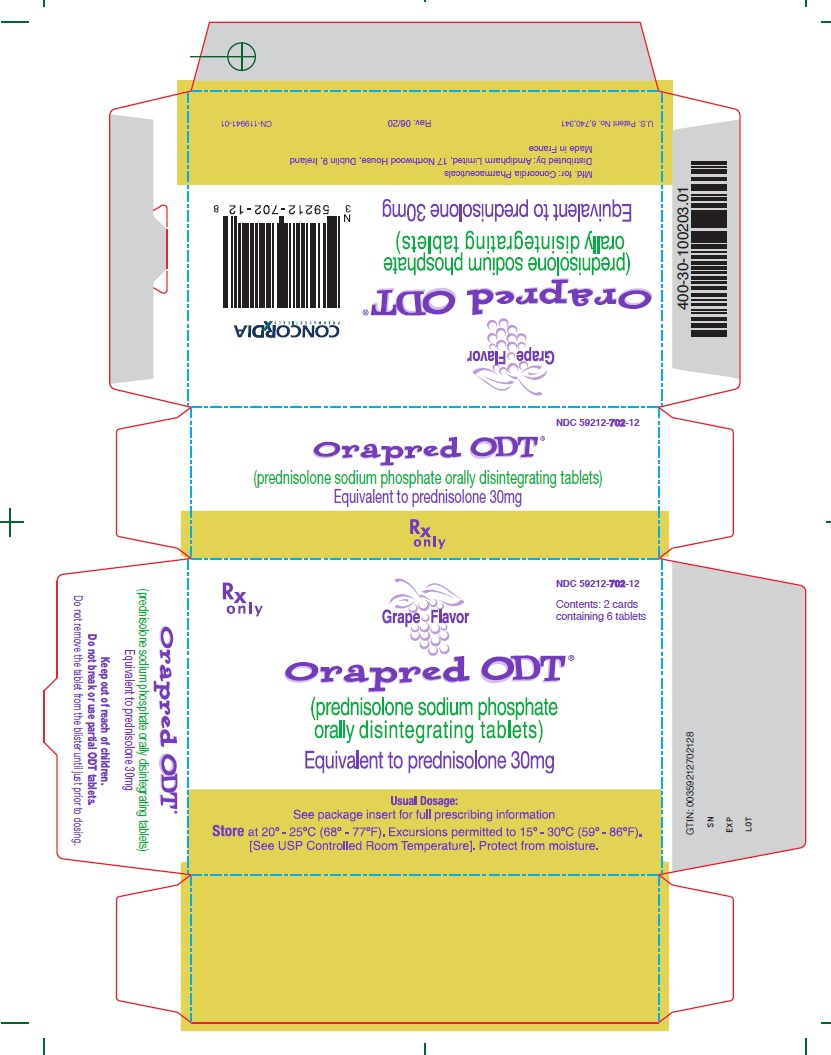
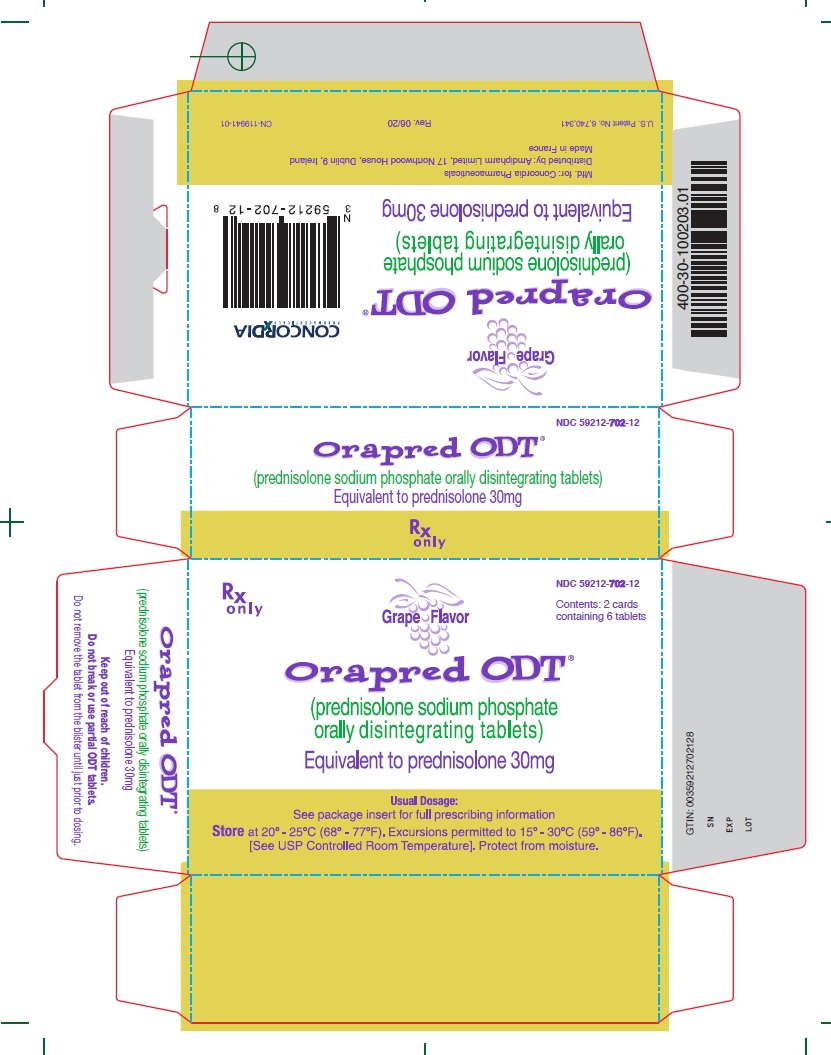
30 mg CartonRx only - NDC 59212-702-12 - Contents: 2 cards containing 6 tablets - Grape Flavor - Orapred ODT® (prednisolone sodium phosphate orally disintegrating tablets) Equivalent to prednisolone 30mg
-
INGREDIENTS AND APPEARANCEProduct Information