Label: ORALONE- triamcinolone acetonide paste
- NDC Code(s): 51672-1335-5, 51672-1335-8
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 14, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
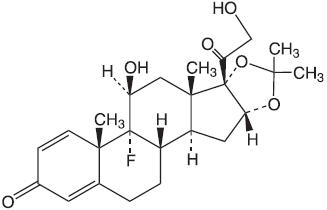
DESCRIPTIONOralone® (Triamcinolone Acetonide Dental Paste USP, 0.1%), contains the corticosteroid triamcinolone acetonide in an adhesive vehicle suitable for application to oral tissues. Triamcinolone ...
-
CLINICAL PHARMACOLOGYLike other topical corticosteroids, triamcinolone acetonide has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical ...
-
INDICATIONS AND USAGEOralone® (Triamcinolone Acetonide Dental Paste USP, 0.1%) is indicated for adjunctive treatment and for the temporary relief of symptoms associated with oral inflammatory lesions and ulcerative ...
-
CONTRAINDICATIONSOralone® is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation; it is also contraindicated in the presence of fungal, viral, or ...
-
PRECAUTIONSGeneral - Oralone® may cause local adverse reactions. If irritation develops, Oralone® should be discontinued and appropriate therapy instituted. Allergic contact sensitization with ...
-
ADVERSE REACTIONSThe following local adverse reactions may occur with corticosteroid-containing dental pastes: burning, itching, irritation, dryness, blistering or peeling not present prior to therapy, perioral ...
-
DOSAGE AND ADMINISTRATIONPress a small dab (about 1/4 inch) to the lesion until a thin film develops. A larger quantity may be required for coverage of some lesions. For optimal results use only enough to coat the lesion ...
-
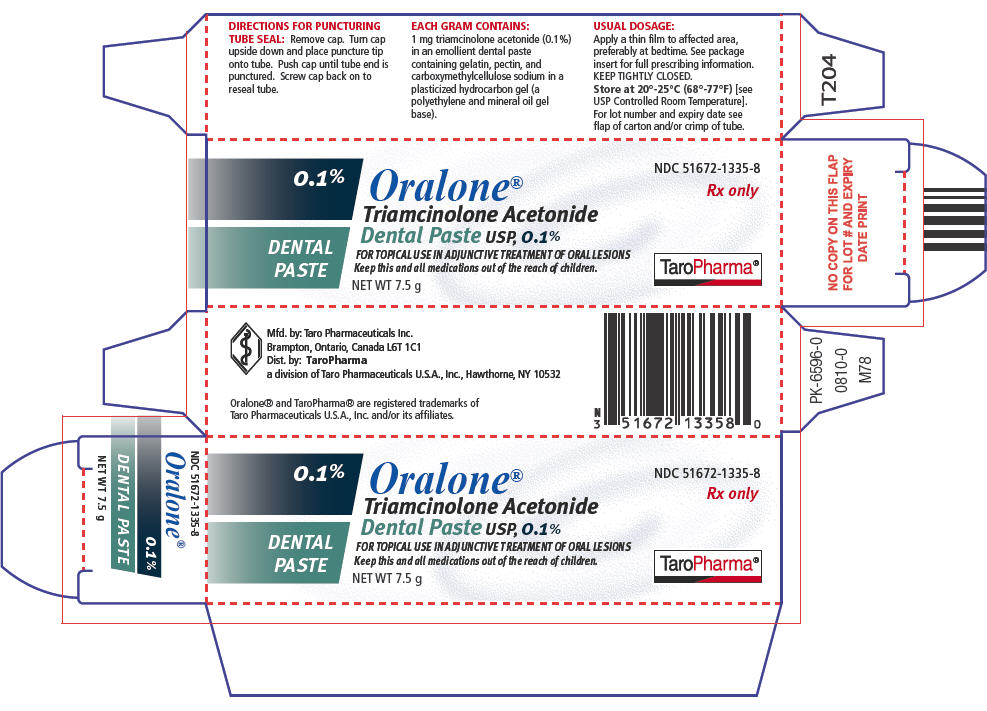
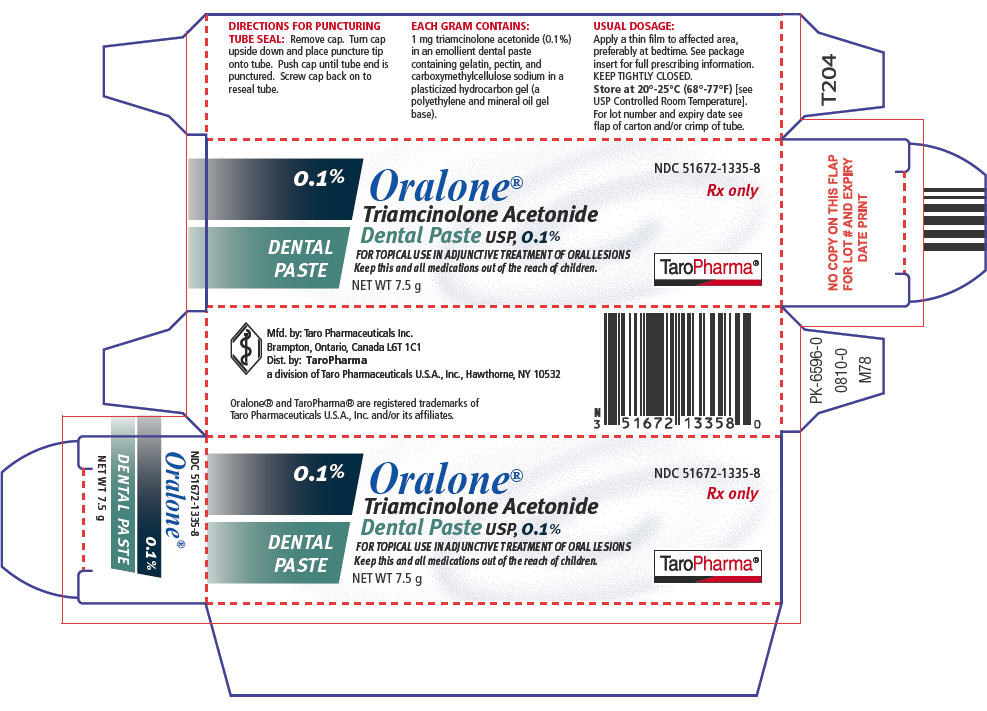
HOW SUPPLIEDOralone® (Triamcinolone Acetonide Dental Paste USP, 0.1%) is supplied in tubes containing 5 g of dental paste (NDC 51672–1335-5) and 7.5 g of dental paste (NDC 51672–1335-8). Storage - Keep ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc. Brampton, Ontario, Canada L6T 1C1 - Dist. by: TaroPharma - a division of Taro Pharmaceuticals U.S.A., Inc. Hawthorne, NY 10532 - Oralone® and TaroPharma® are ...
-
PRINCIPAL DISPLAY PANEL - 7.5 g Carton0.1% DENTAL - PASTE - Oralone® Triamcinolone Acetonide - Dental Paste USP, 0.1% FOR TOPICAL USE IN ADJUNCTIVE TREATMENT OF ORAL LESIONS - Keep this and all medications out of the reach of ...
-
INGREDIENTS AND APPEARANCEProduct Information