Label: ORABLOC- articaine hydrochloride and epinephrine bitartrate injection
- NDC Code(s): 45146-110-01, 45146-110-02, 45146-120-01, 45146-120-02
- Packager: Pierrel S.p.A.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 22, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ORABLOC® safely and effectively. See full prescribing information for ORABLOC®. ORABLOC® (articaine HCl ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEORABLOC is indicated for local, infiltrative, or conductive anesthesia in both simple and complex dental procedures in adults and pediatric patients 4 years of age and older.
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - Table 1 summarizes the recommended dosages of ORABLOC administered by intraoral submucosal infiltration or nerve block for various types of anesthetic dental ...
-
3 DOSAGE FORMS AND STRENGTHSInjection (clear colorless solution), provided in glass cartridges (single-dose) containing (less than a full cartridge or more than one cartridge can be used for an individual ...
-
4 CONTRAINDICATIONSORABLOC is contraindicated in patients who are hypersensitive to products containing sulfites. Products containing sulfites may cause allergic-type reactions including anaphylactic symptoms and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Accidental Intravascular Injection - Accidental intravascular injection of ORABLOC may be associated with convulsions, followed by central nervous system or cardiorespiratory depression and ...
-
6 ADVERSE REACTIONSReactions to articaine are characteristic of those associated with other amide local anesthetics. Adverse reactions to this group of drugs may also result from excessive plasma levels (which may ...
-
7 DRUG INTERACTIONSThe administration of local anesthetic solutions containing epinephrine to patients receiving monoamine oxidase inhibitors, nonselective beta-adrenergic antagonists or tricyclic antidepressants ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects-Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women with articaine with epinephrine. Articaine hydrochloride and ...
-
10 OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local ...
-
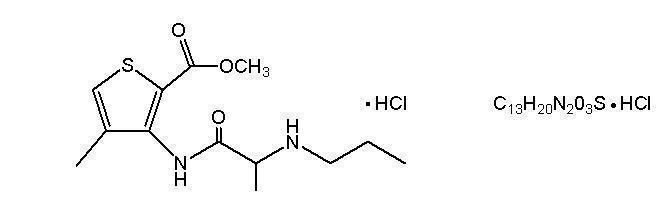
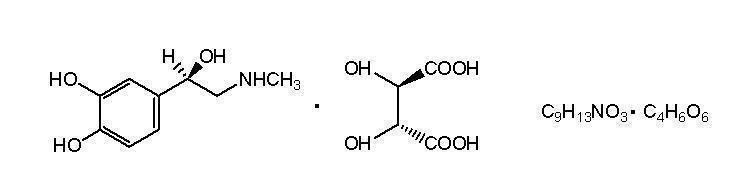
11 DESCRIPTIONORABLOC® (articaine hydrochloride and epinephrine injection), for intraoral submucosal infiltration use, is a sterile, aqueous solution that contains articaine HCl 4% (40mg/mL) and epinephrine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Articaine HCl is an amide local anesthetic. Local anesthetics block the generation and conduction of nerve impulses, presumably by increasing the threshold for ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies to evaluate the carcinogenic potential of articaine HCI in animals have not been conducted. Five standard mutagenicity tests ...
-
14 CLINICAL STUDIESAnother product containing articaine with epinephrine 1:100,000 was studied in three randomized, double-blind, active-controlled trials to evaluate the effectiveness of articaine containing ...
-
15 REFERENCESKaplan, EL, editor. Cardiovascular disease in dental practice. Dallas; American Heart Association; 1986.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGORABLOC® (articaine hydrochloride and epinephrine) injection is a clear, colorless solution available in 1.8 mL single-dose glass cartridges, packaged in boxes of 50 and 100 cartridges in the ...
-
17 PATIENT COUNSELING INFORMATIONLoss of Sensation and Muscle Function - Inform patients in advance of the possibility of temporary loss of sensation and muscle function following infiltration and nerve block injections [see ...
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – Orabloc® (Articaine Hydrochloride 4% and Epinephrine 1:100,000) Injection Cartridge Label
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – Orabloc® (Articaine Hydrochloride 4% and Epinephrine 1:100,000) Injection Carton Label
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – Orabloc® (Articaine Hydrochloride 4% and Epinephrine 1:200,000) Injection Cartridge Label
-
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel – Orabloc® (Articaine Hydrochloride 4% and Epinephrine 1:200,000) Injection Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information