Label: ONPATTRO- patisiran injection, lipid complex
- NDC Code(s): 71336-1000-1
- Packager: Alnylam Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ONPATTRO® safely and effectively. See full prescribing information for ONPATTRO. ONPATTRO (patisiran) lipid complex injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEONPATTRO is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - ONPATTRO should be administered by a healthcare professional. ONPATTRO is administered via intravenous (IV) infusion. Dosing is based on actual body weight. For ...
-
3 DOSAGE FORMS AND STRENGTHSLipid Complex Injection: 10 mg/5 mL (2 mg/mL) white to off-white, opalescent, homogeneous solution in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Infusion-Related Reactions - Infusion-related reactions (IRRs) have been observed in patients treated with ONPATTRO. In clinical studies, all patients received premedication with a ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Infusion-Related Reactions [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ONPATTRO during pregnancy. Physicians are encouraged ...
-
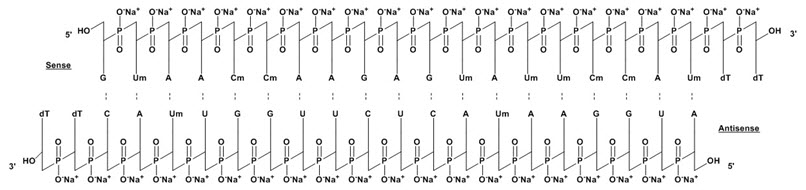
11 DESCRIPTIONONPATTRO contains patisiran, a double-stranded small interfering ribonucleic acid (siRNA), formulated as a lipid complex for delivery to hepatocytes. Patisiran specifically binds to a genetically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Patisiran is a double-stranded siRNA that causes degradation of mutant and wild-type TTR mRNA through RNA interference, which results in a reduction of serum TTR ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Patisiran-LC was not carcinogenic in TgRasH2 mice when administered at intravenous (IV) doses of 0, 0.5, 2, or 6 ...
-
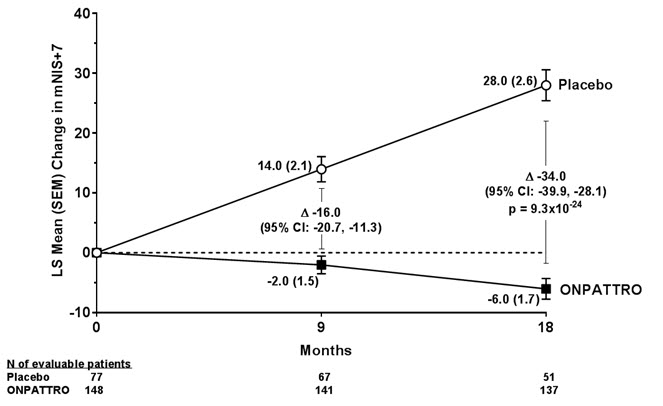
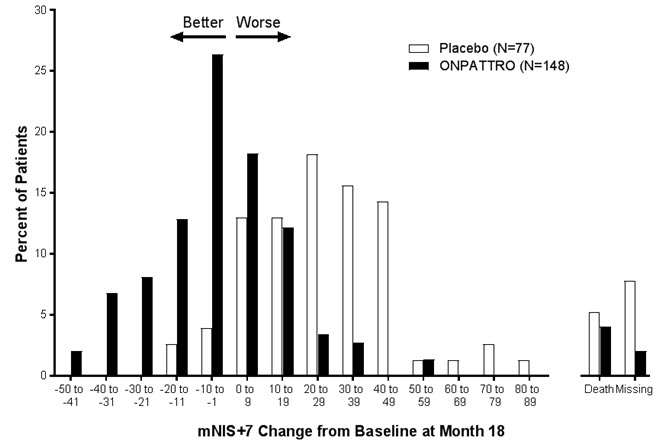
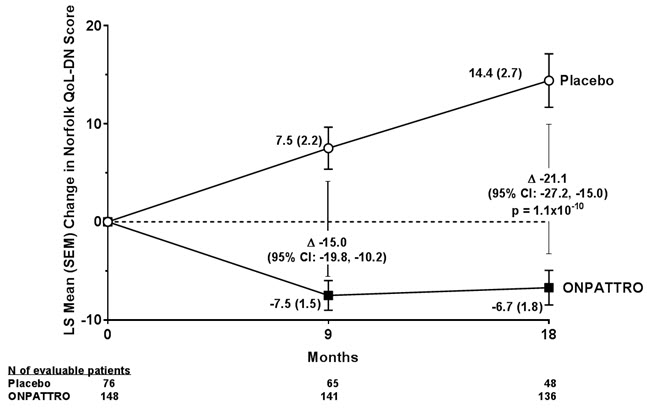
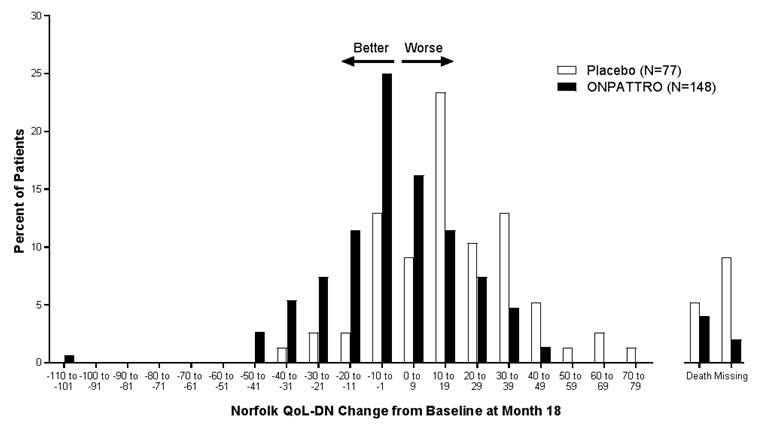
14 CLINICAL STUDIESThe efficacy of ONPATTRO was demonstrated in a randomized, double-blind, placebo-controlled, multicenter clinical trial in adult patients with polyneuropathy caused by hATTR amyloidosis (NCT ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - ONPATTRO is a sterile, preservative-free, white to off-white, opalescent, homogeneous solution for intravenous infusion supplied as a 10 mg/5 mL (2 mg/mL) solution in a ...
-
17 PATIENT COUNSELING INFORMATIONInfusion-Related Reactions - Inform patients about the signs and symptoms of infusion-related reactions (e.g., flushing, dyspnea, chest pain, syncope, rash, increased heart rate, facial edema) ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Alnylam Pharmaceuticals, Inc. 300 Third Street, Cambridge, MA 02142 - By: Ajinomoto Althea, Inc. 11040 Roselle Street, San Diego, CA 92121 - ONPATTRO is a registered trademark of ...
-
PRINCIPAL DISPLAY PANEL - 2 mg/mL Vial CartonNDC 71336-1000-1 - onpattro® (patisiran) lipid complex injection - 10 mg/5 mL - (2 mg/mL) Sterile Solution for - Intravenous Infusion Only - Dilute Before Use - Single-Dose Vial - Discard Unused Portion - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information