Label: ONEXTON- clindamycin phosphate and benzoyl peroxide gel
- NDC Code(s): 0187-3050-35, 0187-3050-50
- Packager: Bausch Health US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 6, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ONEXTON Gel safely and effectively. See full prescribing information for ONEXTON Gel. ONEXTON® (clindamycin phosphate and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEONEXTON® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/3.75% is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.
-
2 DOSAGE AND ADMINISTRATIONBefore applying ONEXTON Gel, wash the face gently with a mild soap, rinse with warm water, and pat the skin dry. Apply a pea-sized amount of ONEXTON Gel to the face once daily. Avoid the eyes ...
-
3 DOSAGE FORMS AND STRENGTHSGel, 1.2%/3.75% Each gram of ONEXTON Gel contains 12 mg (1.2%) clindamycin phosphate, equivalent to 10 mg (1%) clindamycin, and 37.5 mg (3.75%) benzoyl peroxide in a white to off-white, opaque ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - ONEXTON Gel is contraindicated in those individuals who have shown hypersensitivity to clindamycin, benzoyl peroxide, any components of the formulation, or lincomycin ...
-
5 WARNINGS AND PRECAUTIONS5.1 Colitis - Systemic absorption of clindamycin has been demonstrated following topical use of clindamycin. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have ...
-
6 ADVERSE REACTIONSThe following adverse reaction is described in more detail in the Warnings and Precautions section of the label: • Colitis [see Warnings and Precautions (5.1)]. 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Erythromycin - Avoid using ONEXTON Gel in combination with topical or oral erythromycin-containing products due to its clindamycin component. In vitro studies have shown antagonism between ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on ONEXTON Gel use in pregnant women to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or ...
-
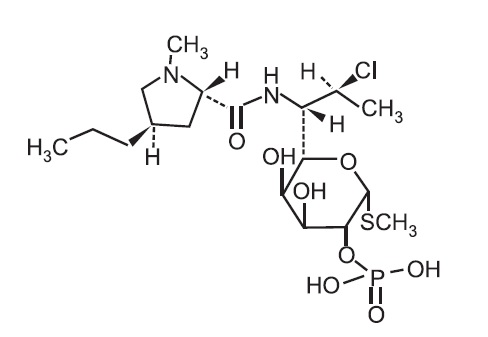
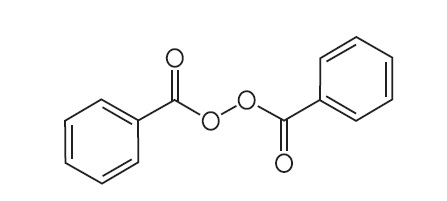
11 DESCRIPTIONONEXTON Gel is a combination product with two active ingredients in a white to off-white, opaque, smooth, aqueous gel formulation intended for topical use. Clindamycin phosphate is a water-soluble ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clindamycin: Clindamycin is a lincosamide antibacterial [see Microbiology (12.4)]. Benzoyl Peroxide: Benzoyl peroxide is an oxidizing agent with bactericidal and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, mutagenicity, and impairment of fertility testing of ONEXTON Gel have not been performed. Benzoyl peroxide has been ...
-
14 CLINICAL STUDIESThe safety and efficacy of once-daily use of ONEXTON Gel was assessed in a 12-week multi-center, randomized, blinded trial in subjects 12 years and older with moderate to severe acne vulgaris ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - ONEXTON Gel, 1.2%/3.75% is a white to off-white smooth gel supplied as: NDC 0187-3050-50 50 g pump - 16.2 Dispensing Instructions for the ...
-
17PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). • Patients who develop allergic reactions, such as severe swelling or shortness of breath, should discontinue ...
-
PATIENT INFORMATION ONEXTON® (ON-EX-TUN) (clindamycin phosphate and benzoyl peroxide) gel, 1.2%/3.75% Important information: ONEXTON Gel is for use on skin only (topical use). Do not use ONEXTON Gel in your mouth ...
-
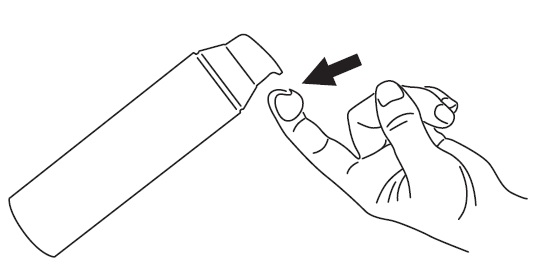
INSTRUCTIONS FOR USE ONEXTON® (ON-EX-TUN) (clindamycin phosphate and benzoyl peroxide) gel, 1.2%/3.75% Important Information: ONEXTON Gel is for use on skin only (topical use). ONEXTON Gel is not for use in your ...
-
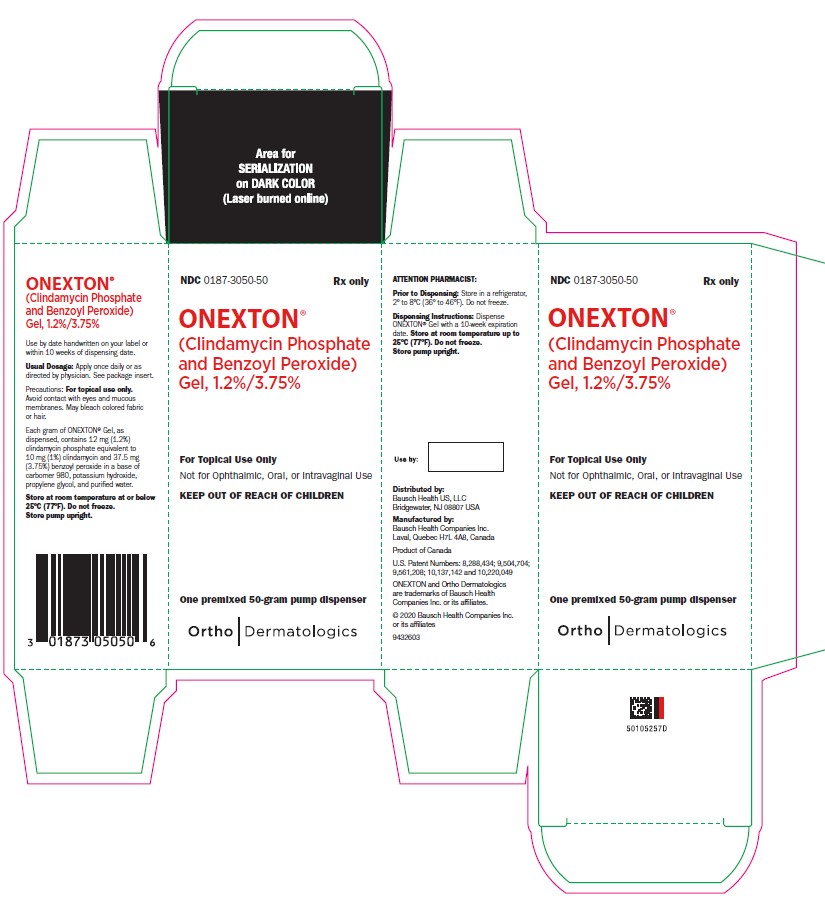
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 50 gram Carton - NDC 0187-3050-50 - Rx only - ONEXTON® (Clindamycin Phosphate and - Benzoyl Peroxide) Gel, 1.2%/3.75% For Topical Use Only - Not for ophthalmic, oral or ...
-
INGREDIENTS AND APPEARANCEProduct Information