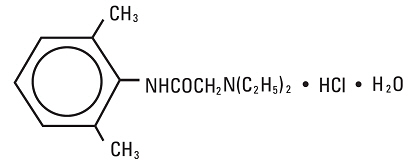Label: OMNITRENIDOL- lidocaine hydrochloride, triamcinolone acetonide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 70529-052-01 - Packager: IT3 Medical LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 24, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
LIDOCAINE HYDROCHLORIDE- lidocaine hydrochloride injection, solutionAQUEOUS SOLUTIONS FOR - INFILTRATION AND NERVE BLOCK - Ampul - Plastic Multiple-dose Fliptop Vial - Glass Teartop Vial - Rx only
-
DESCRIPTIONLidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of lidocaine hydrochloride in water for injection for parenteral administration in various concentrations with ...
-
CLINICAL PHARMACOLOGYMechanism of action - Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic ...
-
INDICATIONS AND USAGELidocaine Hydrochloride Injection, USP is indicated for production of local or regional anesthesia by infiltration techniques such as percutaneous injection and intravenous regional anesthesia by ...
-
CONTRAINDICATIONSLidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type.
-
WARNINGSLIDOCAINE HYDROCHLORIDE INJECTION, FOR INFILTRATION AND NERVE BLOCK, SHOULD BE EMPLOYED ONLY BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE ...
-
PRECAUTIONSGeneral - The safety and effectiveness of lidocaine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be consulted for ...
-
ADVERSE REACTIONSSystemic - Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in ...
-
OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local ...
-
DOSAGE AND ADMINISTRATIONTable 1 (Recommended Dosages) summarizes the recommended volumes and concentrations of Lidocaine Hydrochloride Injection, USP for various types of anesthetic procedures. The dosages suggested in ...
-
HOW SUPPLIED
Lidocaine Hydrochloride Injection, USP is supplied as follows: Unit of Sale Concentration Each - Multiple-dose: 1 MULTI-DOSE - VIAL - 1% 500 mg/50 mL - (10 mg/mL)Plastic Fliptop ...
-
TRIAMCINOLONE ACETONIDE- triamcinolone acetonide injection, suspension, USPNOT FOR USE IN NEONATES - CONTAINS BENZYL ALCOHOL - For Intramuscular or Intra-articular Use Only - NOT FOR INTRAVENOUS, INTRADERMAL, INTRAOCULAR, EPIDURAL, OR INTRATHECAL USE
-
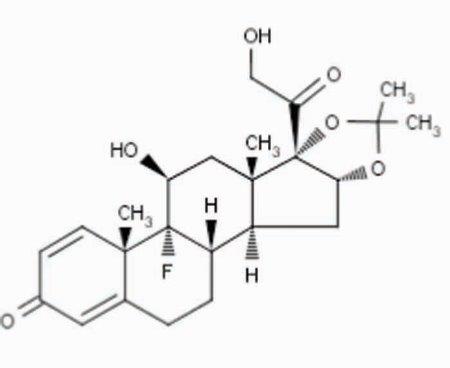
1.1 DESCRIPTIONTriamcinolone acetonide injectable suspension, USP is a synthetic glucocorticoid corticosteroid with anti-inflammatory action. THIS FORMULATION IS SUITABLE FOR INTRAMUSCULAR AND INTRA-ARTICULAR ...
-
1.2 CLINICAL PHARMACOLOGYGlucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Naturally occurring glucocorticoids (hydrocortisone and ...
-
1.3 INDICATIONS AND USAGEIntramuscular - Where oral therapy is not feasible, injectable corticosteroid therapy, including triamcinolone acetonide injectable suspension is indicated for intramuscular use as ...
-
1.4 CONTRAINDICATIONSTriamcinolone acetonide injectable suspension is contraindicated in patients who are hypersensitive to any components of this product (see WARNINGS: General). Intramuscular corticosteroid ...
-
1.5 WARNINGSSerious Neurologic Adverse Reactions with Epidural Administration - Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids (see WARNINGS ...
-
1.6 PRECAUTIONS1.6.1 General - This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the exterior of the vial. The ...
-
1.7 ADVERSE REACTIONS(listed alphabetically under each subsection) The following adverse reactions may be associated with corticosteroid therapy: Allergic reactions: Anaphylaxis including death ...
-
1.8 OVERDOSAGETreatment of acute overdosage is by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy, the dosage of the corticosteroid ...
-
1.9 DOSAGE AND ADMINISTRATIONGeneral - NOTE: CONTAINS BENZYL ALCOHOL (see PRECAUTIONS). The initial dose of triamcinolone acetonide injectable suspension may vary from 2.5 mg to 100 mg per day depending on the specific ...
-
1.10 HOW SUPPLIEDTriamcinolone Acetonide Injectable SuspensionTriamcinolone Acetonide Injectable Suspension, USP is supplied in vials providing 40 mg triamcinolone acetonide per mL. 40 mg/mL, 1 mL single-dose ...
-
MCKESSON ALCOHOL PREP PAD- isopropyl alcohol swabDrug Facts
-
Active ingredientIsopropyl Alcohol 70% v/v
-
PurposeFirst Aid Antiseptic
-
UseFor preparation of the skin prior to an injection
-
WarningsFor external use only - Flammable, keep away from fire or flame - Do not use with electrocautery procedures - Do not use in the eyes - Do not apply to irritated skin - Stop use if pain, irritation ...
-
KEEP OUT OF REACH OF CHILDRENKeep out from reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
-
DirectionsOpen packet - Remove pad - Apply topically as needed to cleanse intended area. Discard after single use.
-
Other informationStore at room temperature 59-86°F (15-30°C) Contents sterile in unopened, undamaged package
-
Inactive ingredientspurified water
-
SPL UNCLASSIFIED SECTIONContents: 1 - 50mL 1% Lidocaine HCL (10mg/mL) 1 - 1mL Triamcinolone Acetonide (40mg/mL) 1 - 5cc Syringe w/ 18-22 Gauge 1-1 1/2 in Needle (Draw) 1 - 27 Gauge 1/2 in Needle (Administer) 1 - 3x3 ...
-
SPL UNCLASSIFIED SECTIONAssembled and Distributed by IT3 Medical, LLC - 190 E Stacy Road; STE 306-298 Allen, TX 75002-8734 - For questions or comments: info@IT3-Medical.com, www.IT3-Medical.com
-
PACKAGING-KIT COMPONENTS LABELING

-
INGREDIENTS AND APPEARANCEProduct Information