Label: OMEGAVEN- fish oil injection, emulsion
- NDC Code(s): 63323-205-00, 63323-205-21, 63323-205-31, 63323-205-50
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OMEGAVEN safely and effectively. See full prescribing information for OMEGAVEN. OMEGAVEN (fish oil triglycerides) injectable ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Omegaven is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC). Limitations of Use: Omegaven is not indicated for ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions - Omegaven can be administered alone or as part of a PN admixture. Omegaven is for central or peripheral intravenous infusion. When administered with ...
-
3 DOSAGE FORMS AND STRENGTHS
Injectable Emulsion: 5 g/50 mL and 10 g/100 mL (0.1 g/mL) sterile, white, homogenous emulsion in a 50-mL and 100-mL single-dose bottle.
-
4 CONTRAINDICATIONS
Use of Omegaven is contraindicated in patients with: Known hypersensitivity to fish or egg protein or to any of the active ingredients or excipients [see Warnings and Precautions (5.2)]. Severe ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants - In the postmarket setting, serious adverse reactions including acute respiratory ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling: Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants ...
-
7 DRUG INTERACTIONS
7.1 Antiplatelet Agents and Anticoagulants - Some published studies have demonstrated prolongation of bleeding time in patients taking antiplatelet agents or anticoagulants and oral omega-3 ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - There are no available data on Omegaven use in pregnant women to establish a drug- associated risk of major birth defects, miscarriage, or adverse maternal or ...
-
10 OVERDOSAGE
In the event of an overdose, serious adverse reactions may occur [see Warnings and Precautions (5.1, 5.4)]. Stop the infusion of Omegaven until triglyceride levels have normalized and any symptoms ...
-
11 DESCRIPTION
Omegaven (fish oil triglycerides) is a sterile, nonpyrogenic, white, homogenous emulsion for intravenous infusion as a supply of calories in patients with PNAC. Each mL of Omegaven contains 0.1 g ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Omegaven provides a biologically utilizable source of calories and essential fatty acids. Fatty acids serve as an important substrate for energy production. The most ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been performed with fish oil triglycerides to evaluate the carcinogenic potential or its effect on fertility. Fish ...
-
14 CLINICAL STUDIES
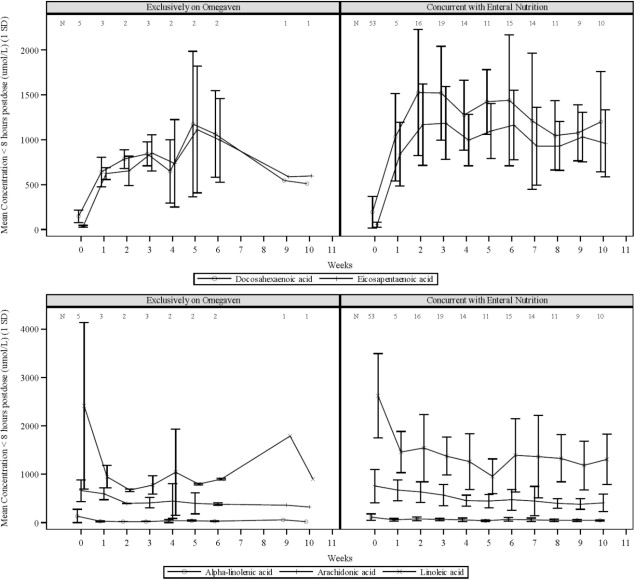
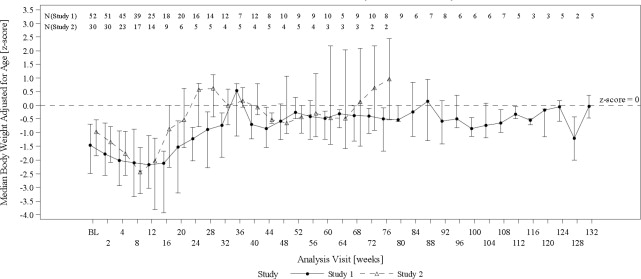
The efficacy of Omegaven was evaluated in two open-label single-center clinical trials (Study 1, NCT00910104, and Study 2, NCT00738101) in pediatric patients with PNAC (defined as direct or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Omegaven (fish oil triglycerides) injectable emulsion, 5 g/50 mL and 10 g/100 mL (0.1 g/mL) is a white, homogenous, sterile emulsion supplied as follows: 50 mL single-dose glass bottle - NDC ...
-
17 PATIENT COUNSELING INFORMATION
When initiating Omegaven administration, discuss the following information: Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants - Inform ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL- PRINCIPAL DISPLAY – Omegaven 50 mL Vial Label - NDC 63323-205-21 255050 - Omegaven - ® (fish oil triglycerides) Injectable emulsion - 5 grams per 50 mL - (0.1 grams per ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL- PRINCIPAL DISPLAY – Omegaven 50 mL Vial Carton Label - NDC 63323-205-50 255050 - Omegaven - ® (fish oil triglycerides) Injectable emulsion - 5 grams per 50 mL - (0.1 grams per ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL- PRINCIPAL DISPLAY – Omegaven 100 mL Vial Label - NDC 63323-205-31 255100 - Omegaven - ® (fish oil triglycerides) Injectable emulsion - 10 grams per 100 mL - (0.1 grams per ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL- PRINCIPAL DISPLAY – Omegaven 100 mL Vial Carton Label - NDC 63323-205-00 255100 - Omegaven - ® (fish oil triglycerides) Injectable emulsion - 10 grams per 100 mL - (0.1 grams per ...
-
INGREDIENTS AND APPEARANCEProduct Information





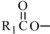 ,
, 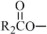 ,
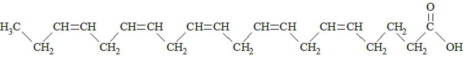
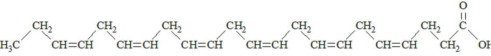
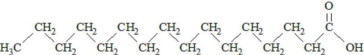
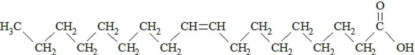
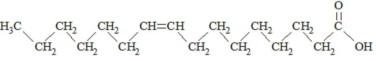
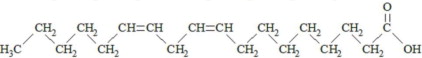
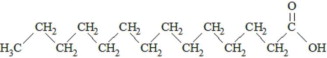
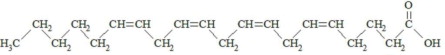
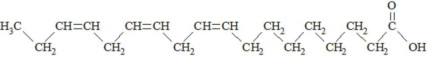
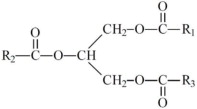
, 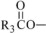 and are long chain acyl groups. Because triglycerides often contain different long chain fatty acids at each position, possible structures can have molecular weights ranging from 700 to 1000 g/mol. The main fatty acid components of the fish oil in Omegaven are EPA (13% to 26%) and DHA (14% to 27%). The fish oil also contains palmitic acid (4% to 12%), oleic acid (4% to 11%), palmitoleic acid (4% to 10%), myristic acid (2% to 7%), and arachidonic acid (0.2% to 2.0%). Additionally, the mean contents of linoleic acid and alpha-linolenic acid are 1.5% and 1.1%, respectively. The fish oil component has a total omega-3 fatty acid content of 40% to 54%. The empirical formula, molecular weight, and chemical structure of the main fatty acid components are:
and are long chain acyl groups. Because triglycerides often contain different long chain fatty acids at each position, possible structures can have molecular weights ranging from 700 to 1000 g/mol. The main fatty acid components of the fish oil in Omegaven are EPA (13% to 26%) and DHA (14% to 27%). The fish oil also contains palmitic acid (4% to 12%), oleic acid (4% to 11%), palmitoleic acid (4% to 10%), myristic acid (2% to 7%), and arachidonic acid (0.2% to 2.0%). Additionally, the mean contents of linoleic acid and alpha-linolenic acid are 1.5% and 1.1%, respectively. The fish oil component has a total omega-3 fatty acid content of 40% to 54%. The empirical formula, molecular weight, and chemical structure of the main fatty acid components are: