Label: NUZYRA- omadacycline injection, powder, lyophilized, for solution
NUZYRA- omadacycline tablet, film coated
- NDC Code(s): 71715-001-01, 71715-001-02, 71715-002-21, 71715-002-23, view more
- Packager: Paratek Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NUZYRA - ®safely and effectively. See full prescribing information for NUZYRA. NUZYRA (omadacycline) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Community-Acquired Bacterial Pneumonia (CABP) NUZYRA is indicated for the treatment of adult patients with community-acquired bacterial pneumonia (CABP) caused by the following susceptible ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - NUZYRA for Injection: Do NOT administer NUZYRA for injection with any solution containing multivalent cations, e.g., calcium and magnesium, through ...
-
3 DOSAGE FORMS AND STRENGTHS3.1 NUZYRA for Injection - Each single-dose vial contains 100 mg omadacycline (equivalent to 131 mg omadacycline tosylate) which must be reconstituted and further diluted prior to intravenous ...
-
4 CONTRAINDICATIONSNUZYRA is contraindicated in patients with known hypersensitivity to omadacycline or tetracycline class antibacterial drugs, or to any of the excipients - [see - Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Mortality Imbalance in Patients with Community-Acquired Bacterial Pneumonia - Mortality imbalance was observed in the CABP clinical trial with eight deaths (2%) occurring in patients treated ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described in greater detail in the Warnings and Precautions section of labeling: Mortality Imbalance in Patients with Community-Acquired ...
-
7 DRUG INTERACTIONS7.1 Anticoagulant Drugs - Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - NUZYRA, like other tetracycline class antibacterial drugs, may cause discoloration of deciduous teeth and reversible inhibition of bone growth when administered ...
-
10 OVERDOSAGENo specific information is available on the treatment of overdosage with NUZYRA. Following a 100 mg single dose intravenous administration of omadacycline, 8.9% of dose is recovered in the ...
-
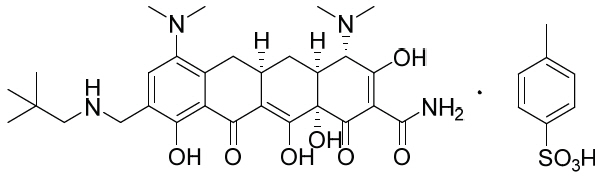
11 DESCRIPTIONNUZYRA contains omadacycline tosylate, an aminomethylcycline which is a semisynthetic derivative of the tetracycline class of antibacterial drugs, for intravenous or oral administration. The ...
-
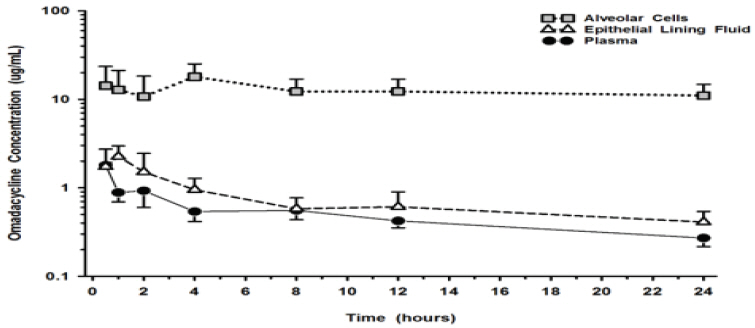
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - NUZYRA is an antibacterial drug - [see - Microbiology (12.4)]. 12.2 Pharmacodynamics - Cardiac Electrophysiology - Based on the nonclinical and clinical ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies with omadacycline have not been conducted. However, there has been evidence of oncogenic ...
-
14 CLINICAL STUDIES14.1 Community-Acquired Bacterial Pneumonia - A total of 774 adults with CABP were randomized in a multinational, double-blind, double-dummy trial (Trial 1, NCT #02531438) comparing NUZYRA to ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - NUZYRA for Injection - NUZYRA for Injection is supplied as a sterile lyophilized powder in a single-dose colorless glass vial, with each vial containing 100 mg of NUZYRA ...
-
17 PATIENT COUNSELING INFORMATIONNausea and Vomiting - Advise patients that nausea and vomiting can be an adverse reaction to NUZYRA. Advise patients that a greater proportion of patients who received the oral loading dose of ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Paratek Pharmaceuticals, Inc. Boston, MA, USA - PARATEK - ®and the hexagon logo are registered trademarks of Paratek Pharmaceuticals, Inc. NUZYRA - ®and its design logo ...
-
PRINCIPAL DISPLAY PANEL - 6 Tablet Blister Pack CartonNDC 71715-002-21 - RX ONLY - once-daily - NUZYRA - ® (omadacycline) 150 mg tablets - Contains 6 tablets
-
PRINCIPAL DISPLAY PANEL - 10 Vial CartonNDC 71715-001-02 - Contains 10 of NDC 71715-001-01 - RX ONLY - NUZYRA - ® (omadacycline) for injection - 100 mg per single-dose vial. Must be reconstituted - and further diluted ...
-
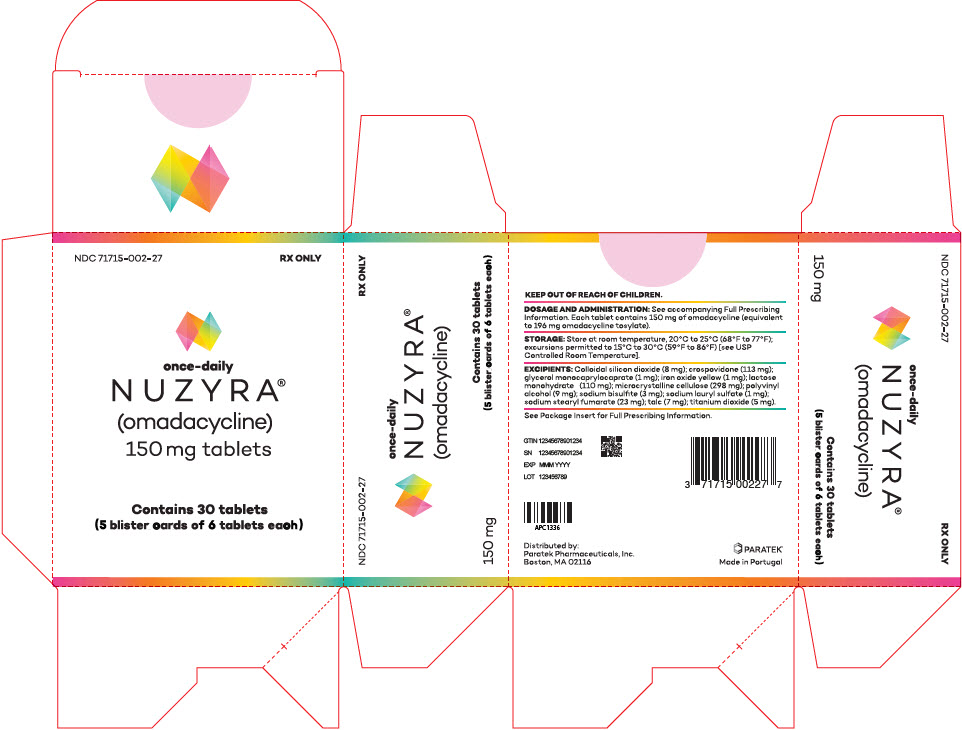
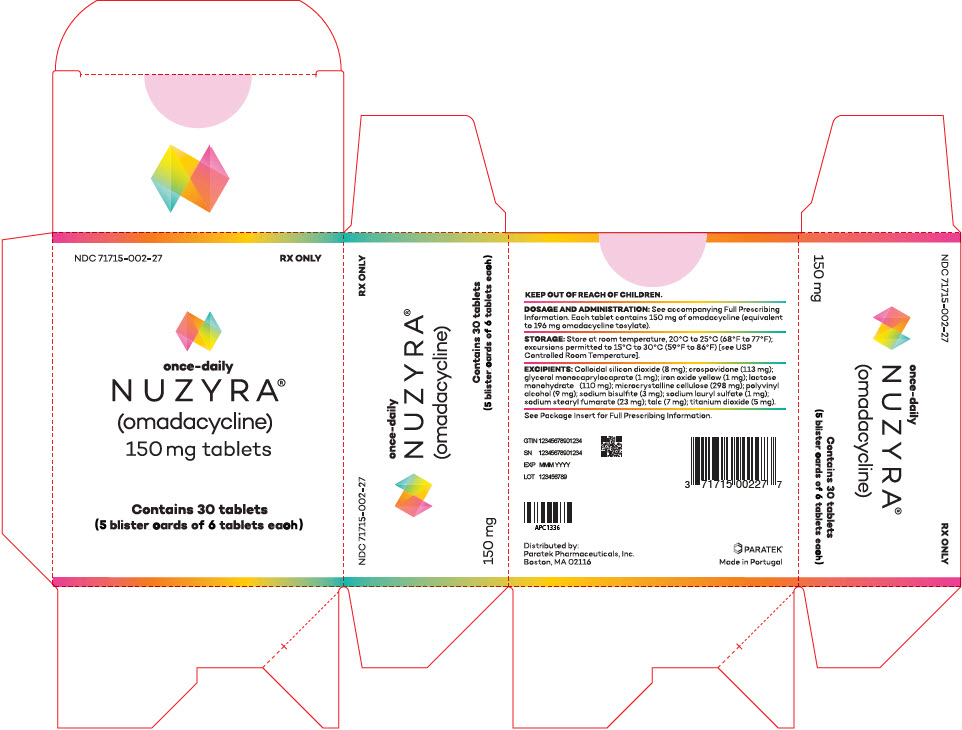
PRINCIPAL DISPLAY PANEL - 30 Tablet Blister Pack CartonNDC 71715-002-27 - RX ONLY - once-daily - NUZYRA - ® (omadacycline) 150 mg tablets - Contains 30 tablets - (5 blister cards of 6 tablets each)
-
INGREDIENTS AND APPEARANCEProduct Information