Label: NYMALIZE- nimodipine solution
- NDC Code(s): 24338-230-05, 24338-230-12, 24338-230-15, 24338-230-30, view more
- Packager: Azurity Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NYMALIZE - ®safely and effectively. See full prescribing information for NYMALIZE. NYMALIZE (nimodipine) oral solution ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENYMALIZE is indicated for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in adult patients with subarachnoid hemorrhage (SAH) from ruptured ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration Instructions - Administer only enterally (e.g., oral, nasogastric tube, or gastric tube route). Do not administer intravenously or by other parenteral routes. For all routes of ...
-
3 DOSAGE FORMS AND STRENGTHSOral Solution (6 mg per mL): 60 mg per 10 mL, pale yellow solution in unit-dose prefilled syringe - 30 mg per 5 mL, pale yellow solution in unit-dose prefilled syringe - 30 mg per 5 mL, pale yellow ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Blood pressure should be carefully monitored during treatment with NYMALIZE. In clinical studies of patients with subarachnoid hemorrhage, about 5% of nimodipine-treated ...
-
6 ADVERSE REACTIONSThe safety and efficacy of NYMALIZE (nimodipine oral solution) in the treatment of patients with SAH is based on adequate and well-controlled studies of nimodipine oral capsules in patients with ...
-
7 DRUG INTERACTIONS7.1 Blood Pressure Lowering Drugs - Nimodipine may increase the blood pressure lowering effect of concomitantly administered anti-hypertensives such as diuretics, beta-blockers, ACE inhibitors ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of NYMALIZE in pregnant women. In animal studies, oral administration of nimodipine ...
-
10 OVERDOSAGEThere have been no reports of overdosage from the oral administration of nimodipine. Symptoms of overdosage would be expected to be related to cardiovascular effects such as excessive peripheral ...
-
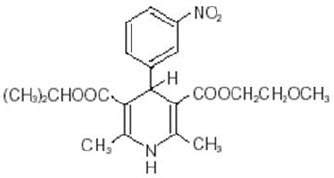
11 DESCRIPTIONNYMALIZE contains nimodipine, a dihydropyridine calcium channel blocker. Nimodipine is isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-( m-nitrophenyl)-3,5-pyridinedicarboxylate. It has a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nimodipine is a dihydropyridine calcium channel blocker. The contractile processes of smooth muscle cells are dependent upon calcium ions, which enter these cells ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a two-year study in rats, the incidences of adenocarcinoma of the uterus and Leydig cell adenoma of the testes ...
-
14 CLINICAL STUDIESThe safety and efficacy of NYMALIZE (nimodipine oral solution) in the treatment of patients with SAH is based on adequate and well-controlled studies of nimodipine oral capsules in patients with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNYMALIZE (nimodipine) Oral Solution 6 mg/mL is a pale yellow solution and is supplied as follows: NDC 24338-260-08: 8 oz. bottle (237 mL) 60 mg/10 mL (6 mg/mL) NDC 24338-260-12: Carton containing ...
-
17 PATIENT COUNSELING INFORMATIONInform patients that the most frequent adverse reaction associated with nimodipine is decreased blood pressure [ see - Warnings and Precautions (5.1)] .Inform them that use of NYMALIZE ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Azurity Pharmaceuticals, Inc. Woburn, MA 01801 - Manufactured by: Delpharm Montréal Inc. Pointe-Claire, QC, Canada - H9R 1B4 - Patent ...
-
PRINCIPAL DISPLAY PANEL - 10 mL Syringe LabelMfg. for Azurity Pharmaceuticals, Inc. Woburn, MA 01801 - Pkg. by Safecor Health - Columbus, OH - NDC 2433826010 - Store at controlled - room temperature, USP. Protect from light ...
-
PRINCIPAL DISPLAY PANEL - 10 mL Syringe Package10 mL - Nymalize - ® (nimodipine) oral solution - 60 mg / 10 mL - For Oral Use Only - Recommended Dosage: See prescribing information. Keep out of reach of children - Package not child ...
-
PRINCIPAL DISPLAY PANEL - 10 mL Syringe Package CartonNDC: 24338-260-12 - Rx Only - Nymalize - ® (nimodipine) oral solution - 60 mg/10 mL - Contains 12 Prefilled - Oral Syringes - For Oral Use Only - Distributed by: azurity - ...
-
PRINCIPAL DISPLAY PANEL - 5 mL Syringe LabelMfg. for Azurity Pharmaceuticals, Inc. Woburn, MA 01801 - Pkg. by Safecor Health - Columbus, OH - NDC 2433823005 - Store at controlled - room temperature, USP. Protect from ...
-
PRINCIPAL DISPLAY PANEL - 5 mL Syringe Package5 mL - Nymalize - ® (nimodipine) oral solution - 30 mg / 5 mL - For Oral Use Only - Recommended Dosage: See prescribing information. Keep out of reach of children - Package not ...
-
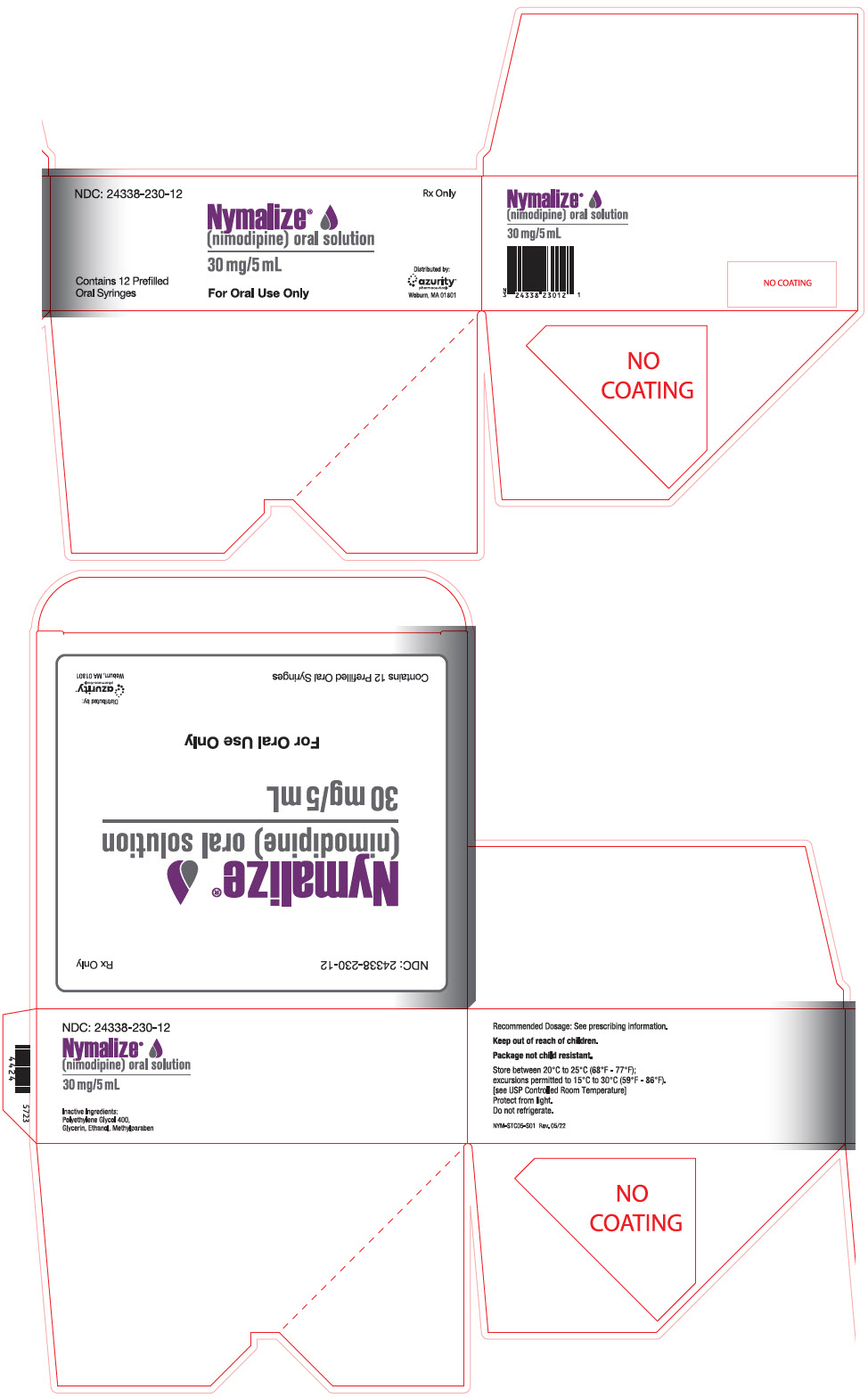
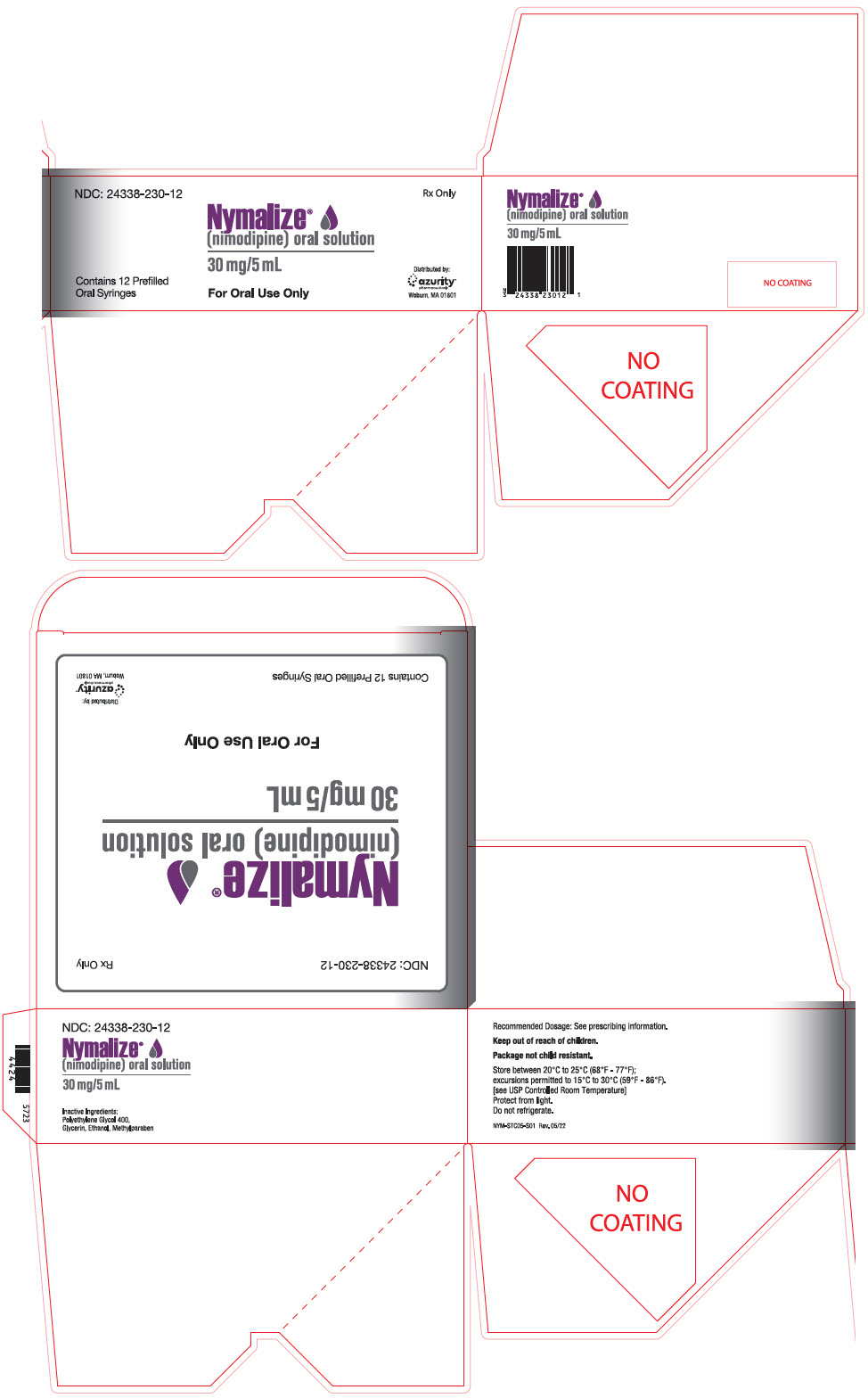
PRINCIPAL DISPLAY PANEL - 5 mL Syringe Package CartonNDC: 24338-230-12 - Rx Only - Nymalize - ® (nimodipine) oral solution - 30 mg/5 mL - Contains 12 Prefilled - Oral Syringes - For Oral Use Only - Distributed by: azurity - ...
-
PRINCIPAL DISPLAY PANEL - 5 mL ENFit® Syringe LabelNDC 24338-230-15 - Rx Only - Nymalize® (nimodipine) oral solution - 30 mg/5 mL - ENFit - ®Syringe - For Oral Use Only
-
PRINCIPAL DISPLAY PANEL - 5 mL ENFit® Syringe Blister LabelNDC 24338-230-15 - Rx Only - Nymalize® (nimodipine) oral solution - 30 mg/5 mL ENFit - ®Syringe - One 5 mL ENFit - ®Syringe - For Oral Use Only - Store between 20°C to 25°C (68°F - 77°F) ...
-
PRINCIPAL DISPLAY PANEL - 5 mL ENFit® Syringe Carton LabelNDC 24338-230-30 - Rx Only - Nymalize® (nimodipine) oral solution - 30 mg/5 mL ENFit® Syringe - Contains 12 Prefilled ENFit - ® Oral Syringes - For Oral Use Only - Recommended Dosage: See ...
-
INGREDIENTS AND APPEARANCEProduct Information