Label: NUTRIARX CREAMPAK- riamcinolone acetonide, dimethicone kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 45802-064-36, 68599-0203-4, 70859-047-01 - Packager: Nucare Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 6, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Dimethicone, 118ml (68599-0203-4)
-
Active ingredientDimethicone 5.0%
-
PurposeSkin Protectant
-
Keep out of reach of childrenIf swallowed, get medical help or contact a Poison Control Center right away.
-
UsesFor the treatment and/or prevention of diaper rash - Temporarily protects and helps relieve chapped or cracked skin
-
WarningsFor external use only - Do not use on - deep or puncture wounds - animal bites - serious burns - When using this product - do not get into eyes - Stop use ...
-
DirectionsCleanse skin with THERA - TM Moisturizing Body Cleanser or THERA - TM Foaming Body Cleanser - Apply cream ...
-
Other informationProtect from freezing. Avoid excessive heat.
-
Inactive ingredientsAleurites Moluccana Seed Oil, Aloe Barbadensis (Aloe Vera) Lead Juice, SAFFLEX - TM (Consisting of: Calcium Pantothenate (Vitamin B - 5) ...
- Trimcinolone Acetonide Cream USP, 80g (45802-064-36)
-
DESCRIPTION
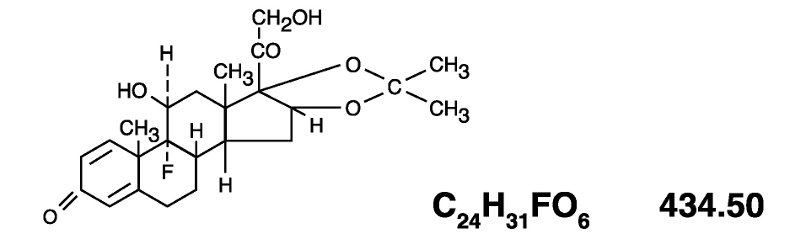
The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. Triamcinolone acetonide is a member of this class. Chemically ...
-
CLINICAL PHARMACOLOGY
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various ...
-
INDICATIONS & USAGE
Triamcinolone acetonide cream is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
-
CONTRAINDICATIONS
Triamcinolone acetonide cream is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
-
PRECAUTIONS
GENERAL PRECAUTIONS - Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome ...
-
ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an ...
-
OVERDOSAGE
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (See PRECAUTIONS).
-
DOSAGE & ADMINISTRATION
Topical corticosteroids are generally applied to the affected area as a thin film from two to three times daily depending on the severity of the condition. Occlusive dressing may be used for ...
-
HOW SUPPLIED
Triamcinolone acetonide cream USP 0.1% is supplied in - 80 g tube NDC 45802-064-36 - Store at 20-25°C (68°-77°F) [see USP Controlled Room Temperature]. Avoid excessive heat ...
-
Silicone TapeSilicone Tape - Uses - • To be applied to wounds or scars as a protective silicone barrier. • As a dressing for abrasions, surgical wounds, donor sites, lacerations, ulcers, skin tears ...
-
Dimethicone
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

-
Trimcinolone Acetonide Cream USP
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

-
NuTriaRX CreamPak
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information