Label: NUCALA- mepolizumab injection, powder, for solution
NUCALA- mepolizumab injection, solution
-
NDC Code(s):
0173-0881-01,
0173-0881-61,
0173-0892-01,
0173-0892-42, view more0173-0892-61, 0173-0892-63, 0173-0904-42
- Packager: GlaxoSmithKline LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 22, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NUCALA safely and effectively. See full prescribing information for NUCALA. NUCALA (mepolizumab) for injection, for subcutaneous ...These highlights do not include all the information needed to use NUCALA safely and effectively. See full prescribing information for NUCALA.
NUCALA (mepolizumab) for injection, for subcutaneous use
NUCALA (mepolizumab) injection, for subcutaneous use
Initial U.S. Approval: 2015RECENT MAJOR CHANGES
INDICATIONS AND USAGE
NUCALA is an interleukin-5 (IL-5) antagonist monoclonal antibody (IgG1 kappa) indicated for:
- •
- Add-on maintenance treatment of adult and pediatric patients aged 6 years and older with severe asthma and with an eosinophilic phenotype. (1.1)
- •
- Add-on maintenance treatment of adult patients aged 18 years and older with chronic rhinosinusitis with nasal polyps (CRSwNP). (1.2)
- •
- Add-on maintenance treatment of adult patients with inadequately controlled chronic obstructive pulmonary disease (COPD) and an eosinophilic phenotype. (1.3)
- •
- The treatment of adult patients with eosinophilic granulomatosis with polyangiitis (EGPA). (1.4)
- •
- The treatment of adult and pediatric patients aged 12 years and older with hypereosinophilic syndrome (HES) for greater than or equal to 6 months without an identifiable non-hematologic secondary cause. (1.5)
Limitations of use: Not for relief of acute bronchospasm or status asthmaticus. (1.1, 1.3)
DOSAGE AND ADMINISTRATION
- •
- Severe asthma in patients aged 12 years and older: 100 mg administered subcutaneously once every 4 weeks. (2.1)
- •
- Severe asthma in patients aged 6 to 11 years: 40 mg administered subcutaneously once every 4 weeks. (2.1)
- •
- CRSwNP: 100 mg administered subcutaneously once every 4 weeks. (2.1)
- •
- COPD: 100 mg administered subcutaneously once every 4 weeks. (2.1)
- •
- EGPA: 300 mg administered subcutaneously once every 4 weeks. (2.1)
- •
- HES: 300 mg administered subcutaneously once every 4 weeks. (2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
History of hypersensitivity to mepolizumab or excipients in the formulation. (4)
WARNINGS AND PRECAUTIONS
- •
- Hypersensitivity reactions (e.g., anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, rash) have occurred after administration of NUCALA. Discontinue NUCALA in the event of a hypersensitivity reaction. (5.1)
- •
- Herpes zoster infections have occurred in patients receiving NUCALA. Consider vaccination if medically appropriate. (5.3)
- •
- Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with NUCALA. Decrease corticosteroids gradually, if appropriate. (5.4)
- •
- Treat patients with pre-existing helminth infections before therapy with NUCALA. If patients become infected while receiving treatment with NUCALA and do not respond to anti-helminth treatment, discontinue NUCALA until parasitic infection resolves. (5.5)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥5%):
- •
- Asthma: Headache, injection site reaction, back pain, and fatigue. (6.1)
- •
- CRSwNP: Oropharyngeal pain and arthralgia. (6.1)
- •
- COPD: Back pain, diarrhea, and cough. (6.1)
- •
- EGPA and HES: Most common adverse reactions are similar to asthma. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Maintenance Treatment of Severe Asthma
1.2 Maintenance Treatment of Chronic Rhinosinusitis with Nasal Polyps
1.3 Maintenance Treatment of Chronic Obstructive Pulmonary Disease
1.4 Eosinophilic Granulomatosis with Polyangiitis
1.5 Hypereosinophilic Syndrome
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Administration of NUCALA for Injection Vial
2.3 Preparation and Administration of NUCALA Injection Prefilled Autoinjector and Prefilled Syringes
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Acute Symptoms of Asthma or Chronic Obstructive Pulmonary Disease or Acute Deteriorating Disease
5.3 Opportunistic Infections: Herpes Zoster
5.4 Reduction of Corticosteroid Dosage
5.5 Parasitic (Helminth) Infection
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Severe Asthma
14.2 Chronic Rhinosinusitis with Nasal Polyps
14.3 Chronic Obstructive Pulmonary Disease
14.4 Eosinophilic Granulomatosis with Polyangiitis
14.5 Hypereosinophilic Syndrome
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE 1.1 Maintenance Treatment of Severe Asthma - NUCALA is indicated for the add-on maintenance treatment of adult and pediatric patients aged 6 years and older with severe asthma and with an ...
1.1 Maintenance Treatment of Severe Asthma
NUCALA is indicated for the add-on maintenance treatment of adult and pediatric patients aged 6 years and older with severe asthma and with an eosinophilic phenotype [see Use in Specific Populations (8.4), Clinical Studies (14.1)].
Limitations of Use
NUCALA is not indicated for the relief of acute bronchospasm or status asthmaticus [see Warnings and Precautions (5.2)].
1.2 Maintenance Treatment of Chronic Rhinosinusitis with Nasal Polyps
NUCALA is indicated for the add-on maintenance treatment of chronic rhinosinusitis with nasal polyps (CRSwNP) in adult patients aged 18 years and older with inadequate response to nasal corticosteroids.
1.3 Maintenance Treatment of Chronic Obstructive Pulmonary Disease
NUCALA is indicated for the add-on maintenance treatment of adult patients with inadequately controlled chronic obstructive pulmonary disease (COPD) and an eosinophilic phenotype.
Limitations of Use
NUCALA is not indicated for the relief of acute bronchospasm [see Warnings and Precautions (5.2)].
1.4 Eosinophilic Granulomatosis with Polyangiitis
NUCALA is indicated for the treatment of adult patients with eosinophilic granulomatosis with polyangiitis (EGPA).
Close1.5 Hypereosinophilic Syndrome
NUCALA is indicated for the treatment of adult and pediatric patients aged 12 years and older with hypereosinophilic syndrome (HES) for greater than or equal to 6 months without an identifiable non-hematologic secondary cause.
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage - NUCALA is for subcutaneous use only, and should be injected into the upper arm, thigh, or abdomen [see Dosage and Administration (2.2, 2.3)]. Table 1. Recommended ...
2.1 Recommended Dosage
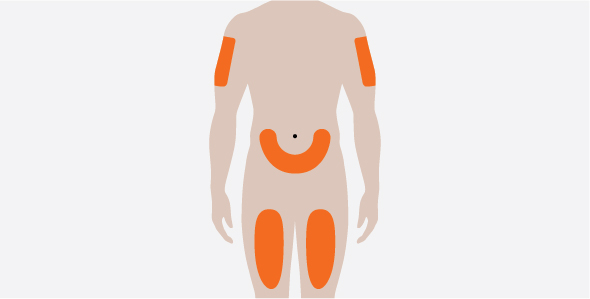
NUCALA is for subcutaneous use only, and should be injected into the upper arm, thigh, or abdomen [see Dosage and Administration (2.2, 2.3)].
Table 1. Recommended Dosage of NUCALA
a 300 mg dose is administered as 3 separate 100 mg dose injections administered at least 5 cm (approximately 2 inches) apart. Indication
Adults
Pediatric Patients
Severe asthma
100 mg every 4 weeks
- •
- 12 to 17 years of age: 100 mg every 4 weeks
- •
- 6 to 11 years of age: 40 mg every 4 weeks
Chronic rhinosinusitis with nasal polyps
100 mg every 4 weeks
Not applicable
Chronic obstructive pulmonary disease
100 mg every 4 weeks
Not applicable
Eosinophilic granulomatosis with polyangiitis
300 mga every 4 weeks
Not applicable
Hypereosinophilic syndrome
300 mga every 4 weeks
12 to 17 years of age: 300 mga every 4 weeks
2.2 Preparation and Administration of NUCALA for Injection Vial
NUCALA for injection should be reconstituted and administered by a healthcare professional. In line with clinical practice, monitoring of patients after administration of biologic agents is recommended [see Warnings and Precautions (5.1)].
Reconstitution Instructions
- 1.
- Reconstitute NUCALA for injection in the vial with 1.2 mL of Sterile Water for Injection, USP, preferably using a 2- or 3-mL syringe and a 21-gauge needle. The reconstituted solution will contain a concentration of 100 mg/mL mepolizumab. Do not mix with other medications.
- 2.
- Direct the stream of Sterile Water for Injection vertically onto the center of the lyophilized powder, which may have a cake-like appearance. Gently swirl the vial for 10 seconds with a circular motion at 15-second intervals until the powder is dissolved.
Note: Do not shake the reconstituted solution during the procedure as this may lead to product foaming or precipitation. Reconstitution is typically complete within 5 minutes after the Sterile Water for Injection has been added, but it may take additional time. - 3.
- If a mechanical reconstitution device (swirler) is used to reconstitute NUCALA for injection, swirl at 450 rpm for no longer than 10 minutes. Alternatively, swirling at 1,000 rpm for no longer than 5 minutes is acceptable.
- 4.
- Visually inspect the reconstituted solution for particulate matter and clarity before use. The solution should be clear to opalescent and colorless to pale yellow or pale brown, essentially particle free. Small air bubbles, however, are expected and acceptable. If particulate matter remains in the solution or if the solution appears cloudy or milky, the solution must not be administered.
- 5.
- If the reconstituted solution is not used immediately:
- •
- store below 30°C (86°F),
- •
- do not freeze, and
- •
- discard if not used within 8 hours of reconstitution.
Administration of 100 mg Dose
- 1.
- For subcutaneous administration, preferably using a 1-mL polypropylene syringe fitted with a disposable 21- to 27-gauge x 0.5-inch (13-mm) needle.
- 2.
- Just before administration, remove 1 mL of reconstituted NUCALA for injection. Do not shake the reconstituted solution during the procedure as this could lead to product foaming or precipitation.
- 3.
- Administer the 1 mL injection (equivalent to 100 mg of mepolizumab) subcutaneously into the upper arm, thigh, or abdomen.
Administration of 40 mg Dose
- 1.
- For subcutaneous administration, preferably using a 1-mL polypropylene syringe fitted with a disposable 21- to 27-gauge x 0.5-inch (13-mm) needle.
- 2.
- Just before administration, remove 0.4 mL of reconstituted NUCALA for injection. Do not shake the reconstituted solution during the procedure as this could lead to product foaming or precipitation.
- 3.
- Administer the 0.4 mL injection (equivalent to 40 mg of mepolizumab) subcutaneously into the upper arm, thigh, or abdomen.
Each vial of NUCALA for injection should be used for a single patient, and any remainder of the contents should be discarded.
Close2.3 Preparation and Administration of NUCALA Injection Prefilled Autoinjector and Prefilled Syringes
NUCALA injection is intended for use under the guidance of a healthcare provider.
The 100 mg/mL prefilled autoinjector and 100 mg/mL prefilled syringe are only for use in adults and adolescents aged 12 years and older. A patient may self-inject, or the patient caregiver may administer NUCALA injection 100 mg/mL subcutaneously after the healthcare provider determines it is appropriate.
The 40 mg/0.4 mL prefilled syringe is only for use in children aged 6 to 11 years and must be administered by the healthcare provider or the patient caregiver. The patient caregiver may administer NUCALA injection 40 mg/0.4 mL subcutaneously after the healthcare provider determines it is appropriate.
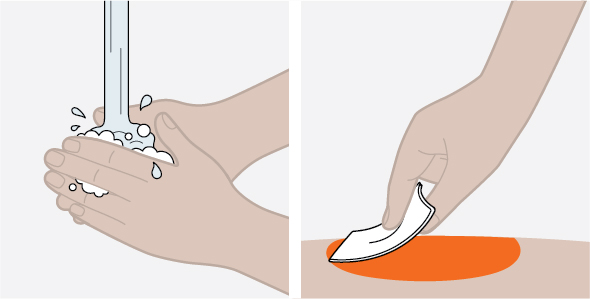
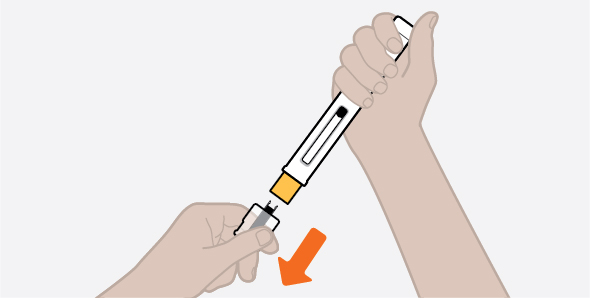
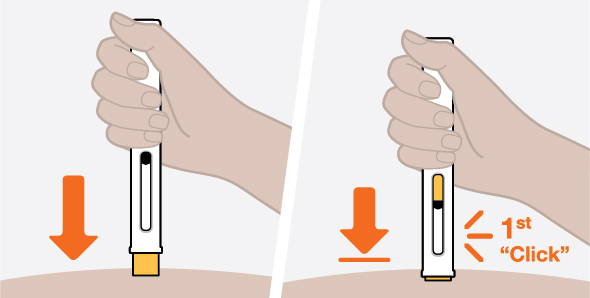
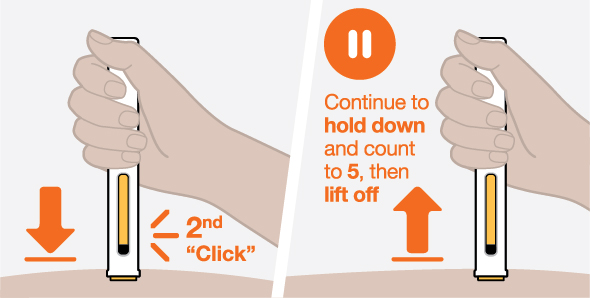
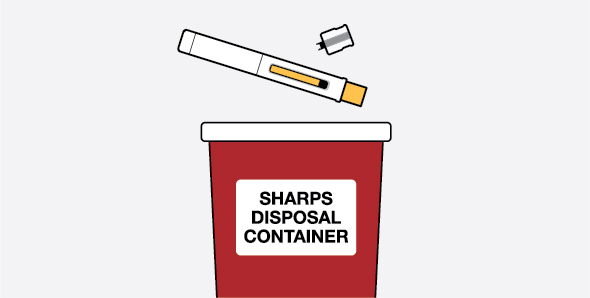
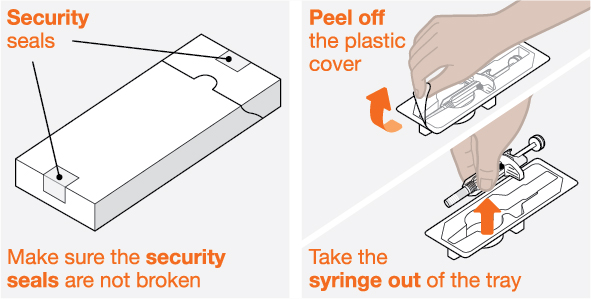
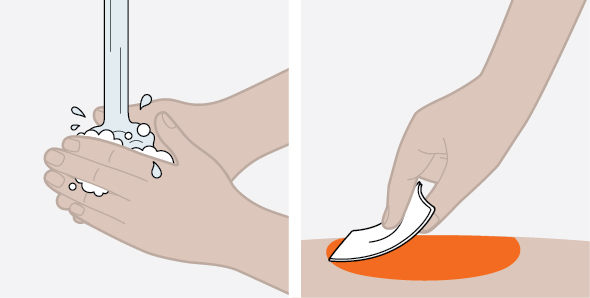
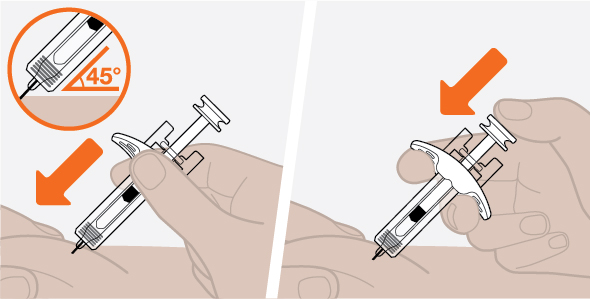
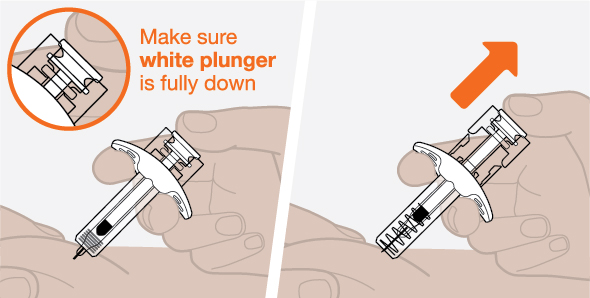
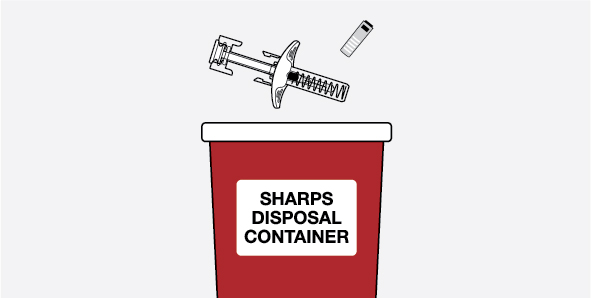
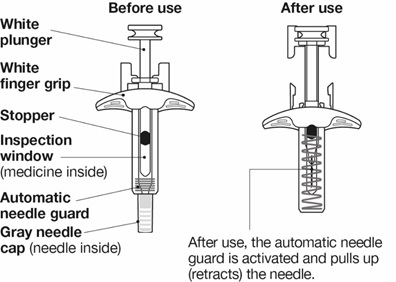
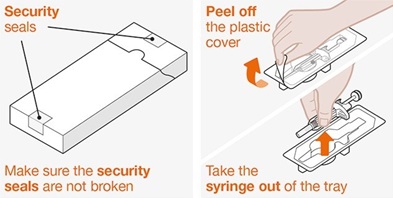
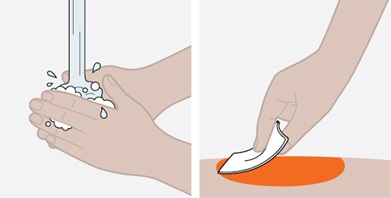
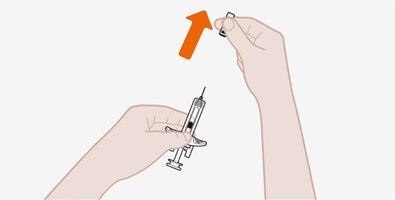
Provide proper training in subcutaneous injection technique and on the preparation and administration of NUCALA injection prior to use [see Instructions for Use].
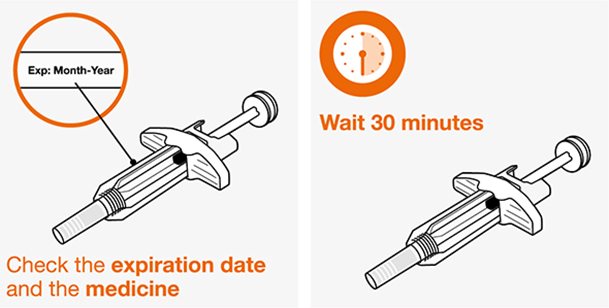
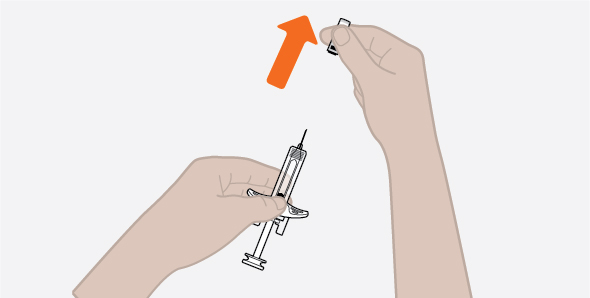
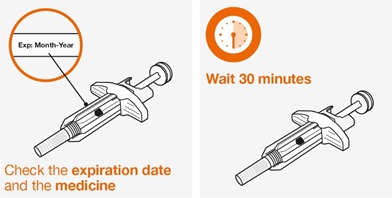
- 1.
- Remove the prefilled autoinjector or prefilled syringe from the refrigerator and allow it to sit at room temperature for 30 minutes prior to injection. Do not warm NUCALA injection in any other way.
- 2.
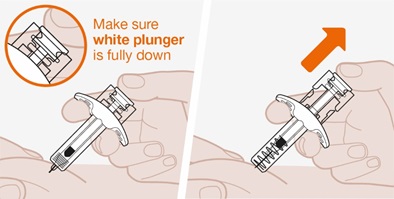
- Prior to administration, visually inspect the window of the prefilled autoinjector or the prefilled syringe for particulate matter or discoloration. NUCALA injection should be clear to opalescent, colorless to pale yellow to pale brown in color. Do not use NUCALA injection if the product exhibits discoloration, cloudiness, or particulate matter. Do not use the NUCALA prefilled autoinjector or prefilled syringe if dropped on a hard surface.
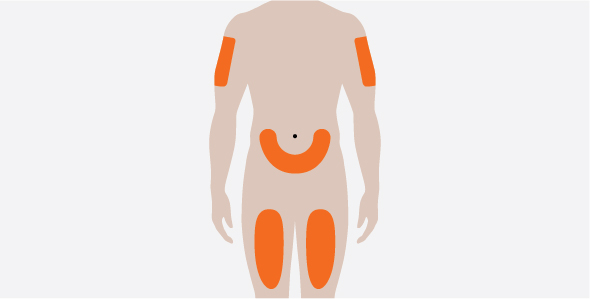
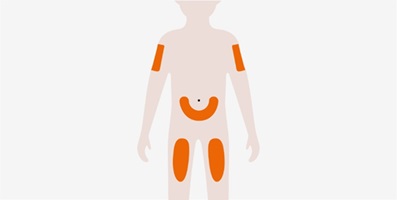
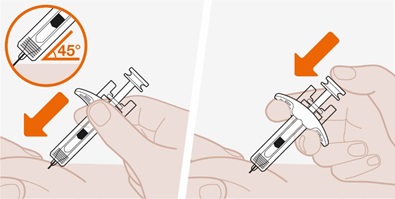
- 3.
- Administer the subcutaneous injection into the thigh or abdomen, avoiding the 5 cm (approximately 2 inches) around the navel. The upper arm can also be used if a caregiver administers the subcutaneous injection.
- 4.
- For use in EGPA and HES, ensure injection sites for each subcutaneous injection are separated by at least 5 cm (approximately 2 inches).
- 5.
- Never give injections into areas where the skin is tender, bruised, red, or hard.
- 6.
- If a dose is missed, administer a dose as soon as possible. Thereafter, the patient can resume dosing on the usual day of administration. If the next dose is already due, then administer as planned.
-
3 DOSAGE FORMS AND STRENGTHS For Injection - • 100 mg white to off-white lyophilized powder in a single-dose vial for reconstitution. Injection - • 100 mg/mL as a clear to opalescent, colorless to pale yellow to pale brown ...
For Injection
- •
- 100 mg white to off-white lyophilized powder in a single-dose vial for reconstitution.
Injection
- •
- 100 mg/mL as a clear to opalescent, colorless to pale yellow to pale brown solution in a single-dose prefilled autoinjector.
- •
- 100 mg/mL as a clear to opalescent, colorless to pale yellow to pale brown solution in a single-dose prefilled glass syringe.
- •
- 40 mg/0.4 mL as a clear to opalescent, colorless to pale yellow to pale brown solution in a single-dose prefilled glass syringe.
-
4 CONTRAINDICATIONS NUCALA is contraindicated in patients with a history of hypersensitivity to mepolizumab or excipients in the formulation [see Warnings and Precautions (5.1), Description (11)].
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypersensitivity Reactions - Hypersensitivity reactions (e.g., anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, rash) have occurred following administration of NUCALA. These ...
5.1 Hypersensitivity Reactions
Hypersensitivity reactions (e.g., anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, rash) have occurred following administration of NUCALA. These reactions generally occur within hours of administration, but in some instances can have a delayed onset (i.e., days). In the event of a hypersensitivity reaction, NUCALA should be discontinued [see Contraindications (4)].
5.2 Acute Symptoms of Asthma or Chronic Obstructive Pulmonary Disease or Acute Deteriorating Disease
NUCALA should not be used to treat acute symptoms or acute exacerbations of asthma or COPD. Do not use NUCALA to treat acute bronchospasm or status asthmaticus. Patients should seek medical advice if their asthma or COPD remains uncontrolled or worsens after initiation of treatment with NUCALA.
5.3 Opportunistic Infections: Herpes Zoster
Herpes zoster has occurred in subjects receiving NUCALA 100 mg in controlled clinical trials [see Adverse Reactions (6.1)]. Consider vaccination if medically appropriate.
5.4 Reduction of Corticosteroid Dosage
Do not discontinue systemic or inhaled corticosteroids (ICS) abruptly upon initiation of therapy with NUCALA. Reductions in corticosteroid dosage, if appropriate, should be gradual and performed under the direct supervision of a physician. Reduction in corticosteroid dosage may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Close5.5 Parasitic (Helminth) Infection
Eosinophils may be involved in the immunological response to some helminth infections. Patients with known parasitic infections were excluded from participation in clinical trials. It is unknown if NUCALA will influence a patient’s response against parasitic infections. Treat patients with pre-existing helminth infections before initiating therapy with NUCALA. If patients become infected while receiving treatment with NUCALA and do not respond to anti-helminth treatment, discontinue treatment with NUCALA until infection resolves.
-
6 ADVERSE REACTIONS The following adverse reactions are described in greater detail in other sections: • Hypersensitivity reactions [see Warnings and Precautions (5.1)] • Opportunistic infections: herpes zoster ...
The following adverse reactions are described in greater detail in other sections:
- •
- Hypersensitivity reactions [see Warnings and Precautions (5.1)]
- •
- Opportunistic infections: herpes zoster [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adult and Adolescent Patients Aged 12 Years and Older with Severe Asthma
A total of 1,327 patients with severe asthma were evaluated in 3 randomized, placebo-controlled, multicenter trials of 24 to 52 weeks’ duration (Severe Asthma Trials DREAM, MENSA, and SIRIUS). Of these, 1,192 had a history of 2 or more exacerbations in the year prior to enrollment despite regular use of high-dose ICS plus additional controller(s) (Severe Asthma Trials DREAM and MENSA), and 135 patients required daily oral corticosteroids (OCS) in addition to regular use of high-dose ICS plus additional controller(s) to maintain asthma control (Severe Asthma Trial SIRIUS). All patients had markers of eosinophilic airway inflammation [see Clinical Studies (14.1)]. Of the patients enrolled, 59% were female, 85% were White, and ages ranged from 12 to 82 years. Mepolizumab was administered subcutaneously or intravenously once every 4 weeks; 263 patients received NUCALA (mepolizumab 100 mg subcutaneously) for at least 24 weeks. Serious adverse events that occurred in more than 1 patient and in a greater percentage of patients receiving NUCALA 100 mg (n = 263) than placebo (n = 257) included 1 event, herpes zoster (2 patients vs. 0 patients, respectively).
The incidence of adverse reactions in the first 24 weeks of treatment in the 2 confirmatory efficacy and safety trials MENSA and SIRIUS with NUCALA 100 mg is shown in Table 2.
Table 2. Adverse Reactions with NUCALA with ≥3% Incidence and More Common than Placebo in Patients with Severe Asthma (MENSA and SIRIUS) Adverse Reaction
NUCALA
(Mepolizumab 100 mg Subcutaneous)
(n = 263)
%
Placebo
(n = 257)
%
Headache
19
18
Injection site reaction
8
3
Back pain
5
4
Fatigue
5
4
Influenza
3
2
Urinary tract infection
3
2
Abdominal pain upper
3
2
Pruritus
3
2
Eczema
3
<1
Muscle spasms
3
<1
52-Week Trial: Adverse reactions from the Severe Asthma Trial DREAM with 52 weeks of treatment with mepolizumab 75 mg intravenously (n = 153) or placebo (n = 155) and with ≥3% incidence and more common than placebo and not shown in Table 2 were: abdominal pain, allergic rhinitis, asthenia, bronchitis, cystitis, dizziness, dyspnea, ear infection, gastroenteritis, lower respiratory tract infection, musculoskeletal pain, nasal congestion, nasopharyngitis, nausea, pharyngitis, pyrexia, rash, toothache, viral infection, viral respiratory tract infection, and vomiting. In addition, 3 cases of herpes zoster occurred in patients receiving mepolizumab 75 mg intravenously compared with 2 patients in the placebo group.
Systemic Reactions, including Hypersensitivity Reactions: In the Severe Asthma Trials DREAM, MENSA, and SIRIUS described above, the percentage of patients who experienced systemic (allergic and non-allergic) reactions was 3% in the group receiving NUCALA 100 mg and 5% in the placebo group. Systemic allergic/hypersensitivity reactions were reported by 1% of patients in the group receiving NUCALA 100 mg and 2% of patients in the placebo group. The most commonly reported manifestations of systemic allergic/hypersensitivity reactions reported in the group receiving NUCALA 100 mg included rash, pruritus, headache, and myalgia. Systemic non-allergic reactions were reported by 2% of patients in the group receiving NUCALA 100 mg and 3% of patients in the placebo group. The most commonly reported manifestations of systemic non‑allergic reactions reported in the group receiving NUCALA 100 mg included rash, flushing, and myalgia. A majority of the systemic reactions in patients receiving NUCALA 100 mg (5/7) were experienced on the day of dosing.
Injection Site Reactions: Injection site reactions (e.g., pain, erythema, swelling, itching, burning sensation) occurred at a rate of 8% in patients receiving NUCALA 100 mg compared with 3% in patients receiving placebo.
Long-Term Safety: Nine hundred ninety-eight patients received NUCALA 100 mg in open-label extension studies, during which additional cases of herpes zoster were reported. The overall adverse event profile has been similar to the severe asthma trials described above.
Adverse Reactions in Pediatric Patients Aged 6 to 11 Years with Severe Asthma
The safety data for NUCALA is based upon 1 open-label clinical trial that enrolled 36 patients with severe asthma aged 6 to 11 years. Patients received 40 mg (for those weighing <40 kg) or 100 mg (for those weighing ≥40 kg) of NUCALA administered subcutaneously once every 4 weeks. Patients received NUCALA for 12 weeks (initial short phase). After a treatment interruption of 8 weeks, 30 patients received NUCALA for a further 52 weeks (long phase). The adverse reaction profile for patients aged 6 to 11 years was similar to that observed in patients aged 12 years and older.
Adverse Reactions in Adult Patients with Chronic Rhinosinusitis with Nasal Polyps
A total of 407 patients with CRSwNP were evaluated in 1 randomized, placebo-controlled, multicenter, 52-week treatment trial. Patients received NUCALA 100 mg or placebo subcutaneously once every 4 weeks. Patients had recurrent CRSwNP with a history of prior surgery and were on nasal corticosteroids for at least 8 weeks prior to screening [see Clinical Studies (14.2)]. Of the patients enrolled, 35% were female, 93% were White, and ages ranged from 18 to 82 years.
Table 3 summarizes adverse reactions that occurred in ≥3% of patients treated with NUCALA and more frequently than in patients treated with placebo in the CRSwNP trial.
Table 3. Adverse Reactions with NUCALA with ≥3% Incidence and More Common than Placebo in Patients with Chronic Rhinosinusitis with Nasal Polyps Adverse Reaction
NUCALA
(Mepolizumab 100 mg
Subcutaneous)
(n = 206)
%
Placebo
(n = 201)
%
Oropharyngeal pain
8
5
Arthralgia
6
2
Abdominal pain upper
3
2
Diarrhea
3
2
Pyrexia
3
2
Nasal dryness
3
<1
Rash
3
<1
Systemic Reactions, including Hypersensitivity Reactions: In the 52-week trial, the percentage of patients who experienced systemic (allergic [type I hypersensitivity] and other) reactions was <1% in the group receiving NUCALA 100 mg and <1% in the placebo group. Systemic allergic (type I hypersensitivity) reactions were reported by <1% of patients in the group receiving NUCALA 100 mg and no patients in the placebo group. The manifestations of systemic allergic (type I hypersensitivity) reactions included urticaria, erythema, and rash and 1 of the 3 reactions occurred on the day of dosing. Other systemic reactions were reported by no patients in the group receiving NUCALA 100 mg and <1% of patients in the placebo group.
Injection Site Reactions: Injection site reactions (e.g., erythema, pruritus) occurred at a rate of 2% in patients receiving NUCALA 100 mg compared with <1% in patients receiving placebo.
Adverse Reactions in Adults with Chronic Obstructive Pulmonary Disease
The safety data below reflects the safety of NUCALA in adults with inadequately controlled COPD and an eosinophilic phenotype. NUCALA was evaluated in a pooled safety population that consisted of 2089 patients with COPD in 3 randomized, placebo-controlled, multicenter trials of 52 to 104 weeks duration, including MATINEE and METREX [see Clinical Studies (14.3)], and Trial 3. Trial 3 enrolled COPD adults with a peripheral blood eosinophil count ≥150 cells/µL at screening or ≥300 cells/µL in the year prior. The pooled safety population received NUCALA 100 mg (n = 1043) or placebo (n = 1046), administered subcutaneously once every 4 weeks, in addition to background triple inhaled therapies (e.g., ICS, long-acting beta agonist [LABA], and long-acting muscarinic antagonist [LAMA]).
Table 4 summarizes adverse reactions that occurred in ≥3% of patients treated with NUCALA and more frequently than in patients treated with placebo in COPD trials.
Table 4. Adverse Reactions with NUCALA with ≥3% Incidence and More Common than Placebo in Patients with Chronic Obstructive Pulmonary Disease in a Pooled Safety Population (MATINEE, METREX, and Trial 3) Adverse Reaction
NUCALA
(Mepolizumab 100 mg Subcutaneous)
(n = 1043)
%
Placebo
(n = 1046)
%
Back pain
7
6
Diarrhea
5
4
Cough
5
4
Oropharyngeal pain
4
2
Urinary tract infection
4
3
Pain in extremity
4
3
Herpes Zoster: In the pooled safety population, 14 (1%) patients in the NUCALA group compared to 7 (0.7%) patients in the placebo group experienced herpes zoster.
Adverse Reactions in Patients with Eosinophilic Granulomatosis with Polyangiitis
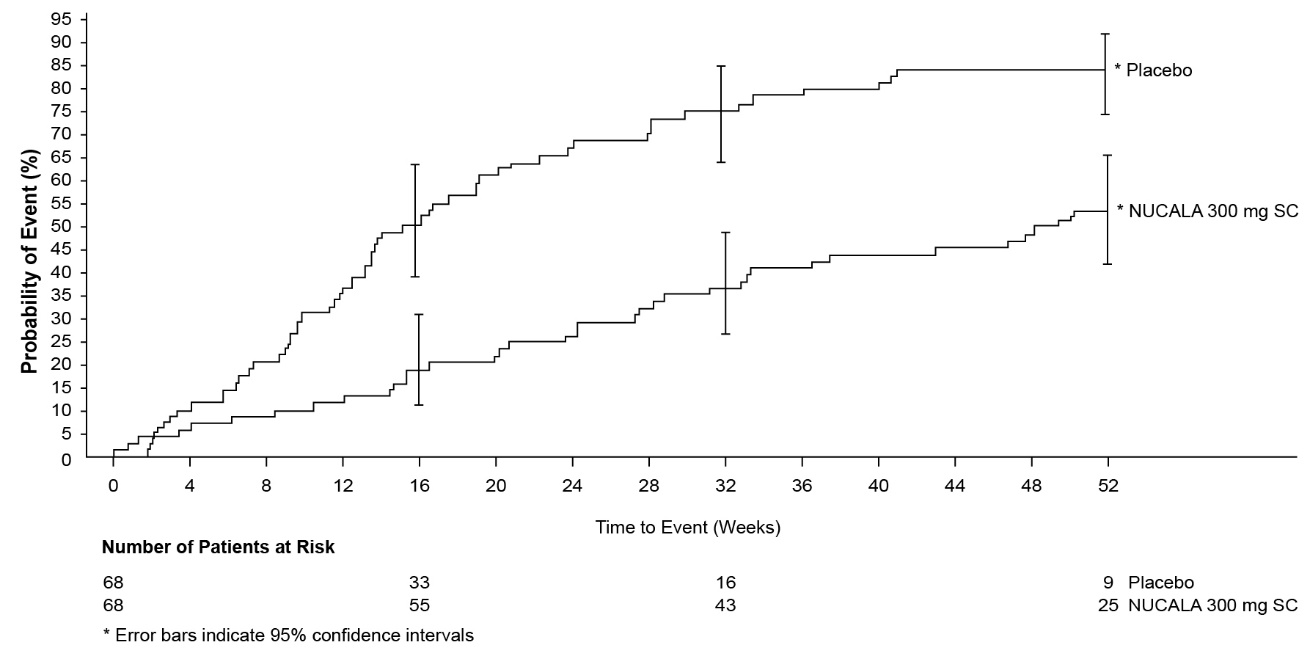
A total of 136 patients with EGPA were evaluated in 1 randomized, placebo-controlled, multicenter, 52-week treatment trial. Patients received 300 mg of NUCALA or placebo subcutaneously once every 4 weeks. Patients enrolled had a diagnosis of EGPA for at least 6 months prior to enrollment with a history of relapsing or refractory disease and were on a stable dosage of oral prednisolone or prednisone of greater than or equal to 7.5 mg/day (but not greater than 50 mg/day) for at least 4 weeks prior to enrollment [see Clinical Studies (14.4)]. Of the patients enrolled, 59% were female, 92% were White, and ages ranged from 20 to 71 years. No additional adverse reactions were identified to those reported in the severe asthma trials.
Systemic Reactions, including Hypersensitivity Reactions: In the 52-week trial, the percentage of patients who experienced systemic (allergic and non‑allergic) reactions was 6% in the group receiving 300 mg of NUCALA and 1% in the placebo group. Systemic allergic/hypersensitivity reactions were reported by 4% of patients in the group receiving 300 mg of NUCALA and 1% of patients in the placebo group. The manifestations of systemic allergic/hypersensitivity reactions reported in the group receiving 300 mg of NUCALA included rash, pruritus, flushing, fatigue, hypertension, warm sensation in trunk and neck, cold extremities, dyspnea, and stridor. Systemic non-allergic reactions were reported by 1 (1%) patient in the group receiving 300 mg of NUCALA and no patients in the placebo group. The reported manifestation of systemic non-allergic reactions reported in the group receiving 300 mg of NUCALA was angioedema. Half of the systemic reactions in patients receiving 300 mg of NUCALA (2/4) were experienced on the day of dosing.
Injection Site Reactions: Injection site reactions (e.g., pain, erythema, swelling) occurred at a rate of 15% in patients receiving 300 mg of NUCALA compared with 13% in patients receiving placebo.
Adverse Reactions in Adult and Adolescent Patients with Hypereosinophilic Syndrome
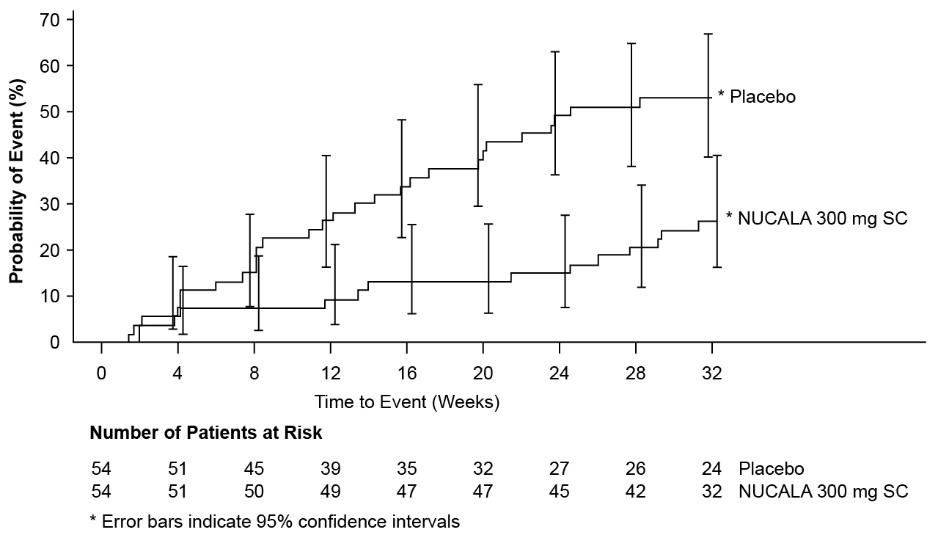
A total of 108 adult and adolescent patients aged 12 years and older with HES were evaluated in a randomized, placebo‑controlled, multicenter, 32-week treatment trial. Patients with non-hematologic secondary HES or FIP1L1‑PDGFRα kinase-positive HES were excluded from the trial. Patients received 300 mg of NUCALA or placebo subcutaneously once every 4 weeks. Patients must have been on a stable dose of background HES therapy for the 4 weeks prior to randomization [see Clinical Studies (14.5)]. Of the patients enrolled, 53% were female, 93% were White, and ages ranged from 12 to 82 years. No additional adverse reactions were identified to those reported in the severe asthma trials.
Systemic Reactions, including Hypersensitivity Reactions: In the trial, no systemic allergic (type I hypersensitivity) reactions were reported. Other systemic reactions were reported by 1 (2%) patient in the group receiving 300 mg of NUCALA and no patients in the placebo group. The reported manifestation of other systemic reaction was multifocal skin reaction experienced on the day of dosing.
Injection Site Reactions: Injection site reactions (e.g., burning, itching) occurred at a rate of 7% in patients receiving 300 mg of NUCALA compared with 4% in patients receiving placebo.
Close6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during post-approval use of NUCALA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to NUCALA or a combination of these factors.
Immune System Disorders
Hypersensitivity reactions, including anaphylaxis.
-
7 DRUG INTERACTIONS Formal drug interaction trials have not been performed with NUCALA.
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - The data on pregnancy exposure are insufficient to inform on drug-associated risk. Monoclonal antibodies, such as mepolizumab, are transported across the placenta ...
8.1 Pregnancy
Risk Summary
The data on pregnancy exposure are insufficient to inform on drug-associated risk. Monoclonal antibodies, such as mepolizumab, are transported across the placenta in a linear fashion as pregnancy progresses; therefore, potential effects on a fetus are likely to be greater during the second and third trimester of pregnancy. In a prenatal and postnatal development study conducted in cynomolgus monkeys, there was no evidence of fetal harm with intravenous administration of mepolizumab throughout pregnancy at doses that produced exposures up to approximately 9 times the exposure at the maximum recommended human dose (MRHD) of 300 mg subcutaneous (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryofetal Risk: In women with poorly or moderately controlled asthma, evidence demonstrates that there is an increased risk of preeclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. The level of asthma control should be closely monitored in pregnant women and treatment adjusted as necessary to maintain optimal control.
Data
Animal Data: In a prenatal and postnatal development study, pregnant cynomolgus monkeys received mepolizumab from gestation Days 20 to 140 at doses that produced exposures up to approximately 9 times that achieved with the MRHD (on an AUC basis with maternal intravenous doses up to 100 mg/kg once every 4 weeks). Mepolizumab did not elicit adverse effects on fetal or neonatal growth (including immune function) up to 9 months after birth. Examinations for internal or skeletal malformations were not performed. Mepolizumab crossed the placenta in cynomolgus monkeys. Concentrations of mepolizumab were approximately 2.4 times higher in infants than in mothers up to Day 178 postpartum. Levels of mepolizumab in milk were ≤0.5% of maternal serum concentration.
In a fertility, early embryonic, and embryofetal development study, pregnant CD-1 mice received an analogous antibody, which inhibits the activity of murine interleukin-5 (IL-5), at an intravenous dose of 50 mg/kg once per week throughout gestation. The analogous antibody was not teratogenic in mice. Embryofetal development of IL-5–deficient mice has been reported to be generally unaffected relative to wild-type mice.
8.2 Lactation
Risk Summary
There is no information regarding the presence of mepolizumab in human milk, the effects on the breastfed infant, or the effects on milk production. However, mepolizumab is a humanized monoclonal antibody (IgG1 kappa), and immunoglobulin G (IgG) is present in human milk in small amounts. Mepolizumab was present in the milk of cynomolgus monkeys postpartum following dosing during pregnancy [see Use in Specific Populations (8.1)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NUCALA and any potential adverse effects on the breastfed infant from mepolizumab or from the underlying maternal condition.
8.4 Pediatric Use
Severe Asthma
The safety and effectiveness of NUCALA for severe asthma, and with an eosinophilic phenotype, have been established in pediatric patients aged 6 years and older.
Use of NUCALA in adolescents aged 12 to 17 years is supported by evidence from adequate and well-controlled trials in adults and adolescents. A total of 28 adolescents aged 12 to 17 years with severe asthma were enrolled in the Phase 3 asthma trials. Of these, 25 were enrolled in the 32-week exacerbation trial (MENSA) and had a mean age of 14.8 years. Patients had a history of 2 or more exacerbations in the previous year despite regular use of medium- or high-dose ICS plus additional controller(s) with or without OCS and had blood eosinophils of ≥150 cells/mcL at screening or ≥300 cells/mcL within 12 months prior to enrollment. [See Clinical Studies (14.1).] Patients had a reduction in the rate of exacerbations that trended in favor of NUCALA. Of the 19 adolescents who received NUCALA, 9 received 100 mg and the mean apparent clearance in these patients was 35% less than that of adults. The safety profile observed in adolescents was generally similar to that of the overall population in the Phase 3 studies [see Adverse Reactions (6.1)].
Use of NUCALA in pediatric patients aged 6 to 11 years with severe asthma, and with an eosinophilic phenotype, is supported by evidence from adequate and well-controlled trials in adults and adolescents with additional pharmacokinetic, pharmacodynamic, and safety data in children aged 6 to 11 years. A single open-label clinical trial was conducted in 36 children aged 6 to 11 years (mean age: 8.6 years, 31% female) with severe asthma. Enrollment criteria were the same as for adolescents in the 32-week exacerbation trial (MENSA). Based upon the pharmacokinetic data from this trial, a subcutaneous dose of 40 mg every 4 weeks was determined to have similar exposure to adults and adolescents administered a subcutaneous dose of 100 mg [see Clinical Pharmacology (12.3)].
The effectiveness of NUCALA in pediatric patients aged 6 to 11 years is extrapolated from efficacy in adults and adolescents with support from pharmacokinetic analyses showing similar drug exposure levels for 40 mg administered subcutaneously every 4 weeks in children aged 6 to 11 years compared with adults and adolescents [see Clinical Pharmacology (12.3)]. The safety profile and pharmacodynamic response observed in this trial for children aged 6 to 11 years were similar to that seen in adults and adolescents [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)].
The safety and effectiveness in pediatric patients aged younger than 6 years with severe asthma have not been established.
Chronic Rhinosinusitis with Nasal Polyps
The safety and effectiveness in patients aged younger than 18 years with CRSwNP have not been established.
Chronic Obstructive Pulmonary Disease
The safety and effectiveness in pediatric patients aged younger than 18 years with COPD have not been established. COPD is largely a disease of adult patients.
Eosinophilic Granulomatosis with Polyangiitis
The safety and effectiveness in patients aged younger than 18 years with EGPA have not been established.
Hypereosinophilic Syndrome
The safety and effectiveness of NUCALA for HES have been established in adolescent patients aged 12 years and older. The safety and effectiveness in pediatric patients aged younger than 12 years with HES have not been established.
Use of NUCALA for this indication is supported by evidence from an adequate and well-controlled study (NCT02836496) in adults and adolescents and an open-label extension study (NCT03306043). One adolescent received NUCALA during the controlled study and this patient and an additional 3 adolescents received NUCALA during the open-label extension study [see Clinical Studies (14.5)]. The 1 adolescent treated with NUCALA in the 32-week trial did not have a HES flare or an adverse event reported. All adolescents received 300 mg of NUCALA for 20 weeks in the open-label extension.
Close8.5 Geriatric Use
Of the total number of patients treated with NUCALA (n = 4106) for severe asthma, HES, EGPA, CRSwNP, and COPD, 1073 (26%) were 65 years of age and older and 241 (6%) were 75 years of age and older [see Clinical Studies (14)]. No overall differences in safety and effectiveness were observed between these patients and younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity in some older individuals cannot be ruled out.
-
10 OVERDOSAGE There is no specific treatment for an overdose with mepolizumab. If overdose occurs, the patient should be treated supportively with appropriate monitoring as necessary. Consider contacting the ...
There is no specific treatment for an overdose with mepolizumab. If overdose occurs, the patient should be treated supportively with appropriate monitoring as necessary. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
Close -
11 DESCRIPTION Mepolizumab is a humanized IL-5 antagonist monoclonal antibody. Mepolizumab is produced by recombinant DNA technology in Chinese hamster ovary cells. Mepolizumab has a molecular weight of ...
Mepolizumab is a humanized IL-5 antagonist monoclonal antibody. Mepolizumab is produced by recombinant DNA technology in Chinese hamster ovary cells. Mepolizumab has a molecular weight of approximately 149 kDa.
NUCALA for injection is a sterile, preservative-free, white to off-white, lyophilized powder in a single-dose vial for subcutaneous injection after reconstitution. Upon reconstitution with 1.2 mL of Sterile Water for Injection, USP, the resulting concentration is 100 mg/mL and delivers 1 mL [see Dosage and Administration (2.2)]. Each vial delivers 100 mg of mepolizumab, polysorbate 80 (0.67 mg), sodium phosphate dibasic heptahydrate (7.14 mg), and sucrose (160 mg), with a pH of 7.0.
The vial stopper is not made with natural rubber latex.
NUCALA injection is a sterile, preservative-free, clear to opalescent, colorless to pale yellow to pale brown solution for subcutaneous use.
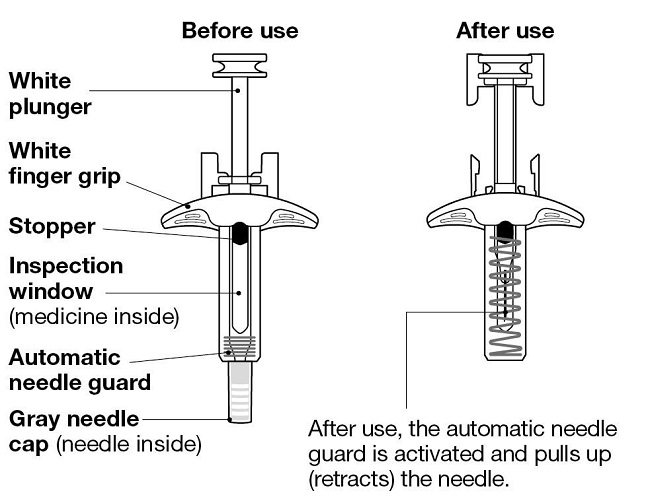
NUCALA injection is supplied in a single-dose, 1-mL, prefilled autoinjector with a fixed 29‑gauge, half-inch needle and in a single-dose, 1-mL, prefilled syringe with a fixed 29‑gauge, half-inch needle with a needle guard. Each 1 mL delivers 100 mg mepolizumab, citric acid monohydrate (0.95 mg), EDTA disodium dihydrate (0.019 mg), polysorbate 80 (0.20 mg), sodium phosphate dibasic heptahydrate (4.16 mg), and sucrose (120 mg), with a pH of 6.3.
NUCALA injection is supplied in a single-dose, 0.4-mL, prefilled syringe with a fixed 29-gauge, half-inch needle with a needle guard. Each 0.4 mL delivers 40 mg mepolizumab, citric acid monohydrate (0.38 mg), EDTA disodium dihydrate (0.0074 mg), polysorbate 80 (0.08 mg), sodium phosphate dibasic heptahydrate (1.66 mg), and sucrose (48 mg), with a pH of 6.3.
The prefilled autoinjector and prefilled syringe are not made with natural rubber latex.
Close -
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Mepolizumab is an IL-5 antagonist (IgG1 kappa). IL-5 is the major cytokine responsible for the growth and differentiation, recruitment, activation, and survival of ...
12.1 Mechanism of Action
Mepolizumab is an IL-5 antagonist (IgG1 kappa). IL-5 is the major cytokine responsible for the growth and differentiation, recruitment, activation, and survival of eosinophils. Mepolizumab binds to IL-5 with a dissociation constant of 100 pM, inhibiting the bioactivity of IL-5 by blocking its binding to the alpha chain of the IL-5 receptor complex expressed on the eosinophil cell surface. Inflammation is an important component in the pathogenesis of asthma, CRSwNP, COPD, EGPA, and HES. Multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) are involved in inflammation. Mepolizumab, by inhibiting IL-5 signaling, reduces the production and survival of eosinophils; however, the mechanism of mepolizumab action in asthma, CRSwNP, COPD, EGPA, and HES has not been definitively established.
12.2 Pharmacodynamics
The pharmacodynamic response (blood eosinophil reduction) following repeat doses of mepolizumab administered subcutaneously or intravenously was evaluated in adult subjects with asthma and blood eosinophil levels >200 cells/mcL. Subjects received 1 of 4 mepolizumab treatments (administered every 28 days for a total of 3 doses): 12.5 mg subcutaneous, 125 mg subcutaneous, 250 mg subcutaneous, or 75 mg intravenous. Sixty-six of the 70 randomized subjects completed the trial. Compared with baseline levels, blood eosinophils decreased in a dose‑dependent manner. A reduction in blood eosinophil levels was observed in all treatment groups by Day 3 (48 hours post-dose). On Day 84 (4 weeks post-last dose), the observed geometric mean reduction from baseline in blood eosinophils was 64%, 78%, 84%, and 90% in the 12.5-mg subcutaneous, 75-mg intravenous, 125-mg subcutaneous, and 250-mg subcutaneous treatment groups, respectively. The model-predicted subcutaneous doses providing 50% and 90% of maximal reduction of blood eosinophils at Day 84 were estimated to be 11 and 99 mg, respectively. These results, along with the clinical efficacy data from the dose-ranging exacerbation DREAM Trial in adult and adolescent subjects with severe asthma supported the evaluation of mepolizumab 75 mg intravenous and 100 mg subcutaneous in the confirmatory severe asthma trials [see Clinical Studies (14.1)]. Following subcutaneous administration of mepolizumab 100 mg every 4 weeks for 32 weeks in adult and adolescent subjects with severe asthma (MENSA Trial), blood eosinophils were reduced to a geometric mean count of 40 cells/mcL, which corresponds to a geometric mean reduction of 84% compared with placebo.
The pharmacodynamic response (blood eosinophil reduction) was also evaluated in children aged 6 to 11 years with severe asthma. Following subcutaneous administration of mepolizumab 40 mg every 4 weeks for 52 weeks, blood eosinophils were reduced to a geometric mean count of 48 cells/mcL. This corresponds to a geometric mean reduction from baseline of 85%.
The magnitude of reduction in adults, adolescents, and children with severe asthma was observed within 4 weeks of treatment and was maintained throughout the treatment periods.
Following subcutaneous administration of NUCALA 100 mg every 4 weeks (for CRSwNP or COPD), or 300 mg every 4 weeks (for EGPA or HES), blood eosinophils were reduced to a similar magnitude to that observed in patients with severe asthma compared to placebo.
For adults with CRSwNP, following subcutaneous administration of mepolizumab 100 mg every 4 weeks for 52 weeks, blood eosinophils were reduced to a geometric mean count of 60 cells/mcL. There was a geometric mean reduction of 83% compared with placebo. This magnitude of reduction was observed within 4 weeks of treatment and was maintained throughout the treatment period [see Clinical Studies (14.2)].
For adults with COPD, following subcutaneous administration of mepolizumab 100 mg every 4 weeks for 52 to 104 weeks, blood eosinophils were reduced to a geometric mean count of 50‑60 cells/mcL. There was a geometric mean reduction of approximately 79% compared with placebo, and this magnitude of reduction was observed within 4 weeks of treatment and was maintained throughout the treatment period [see Clinical Studies (14.3)].
For adults with EGPA, following subcutaneous administration of mepolizumab 300 mg every 4 weeks for 52 weeks, blood eosinophils were reduced to a geometric mean count of 38 cells/mcL. There was a geometric mean reduction of 83% compared with placebo, and this magnitude of reduction was observed within 4 weeks of treatment [see Clinical Studies (14.4)].
For adults and pediatric patients aged 12 years and older with HES, following subcutaneous administration of mepolizumab 300 mg every 4 weeks for 32 weeks, blood eosinophils were reduced to a geometric mean count of 70 cells/mcL. There was a geometric mean reduction of 92% compared with placebo [see Clinical Studies (14.5)].
12.3 Pharmacokinetics
Following subcutaneous dosing in adult subjects with asthma, mepolizumab exhibited approximately dose‑proportional pharmacokinetics over a dose range of 12.5 to 250 mg. The pharmacokinetic properties of mepolizumab observed in subjects with CRSwNP (adults), COPD (adults), EGPA (adults), or HES (adults and adolescents) were similar to the pharmacokinetic properties observed in subjects with severe asthma (adults and adolescents).
Subcutaneous administration of mepolizumab 300 mg had approximately 3 times the systemic exposure of mepolizumab 100 mg.
Absorption
Following 100-mg subcutaneous administration in the upper arm of adult and adolescent subjects with asthma, the bioavailability of mepolizumab was estimated to be approximately 80%.
Following repeat subcutaneous administration once every 4 weeks, there was approximately a 2-fold accumulation at steady state.
Distribution
The population central volume of distribution of mepolizumab in adult subjects with asthma is estimated to be 3.6 L for a 70-kg individual.
Elimination
Following subcutaneous administration of mepolizumab in adult subjects with asthma, the mean terminal half-life (t1/2) ranged from 16 to 22 days. The population apparent systemic clearance of mepolizumab in adult and adolescent subjects with asthma is estimated to be 0.28 L/day for a 70-kg individual.
Metabolism: Mepolizumab is a humanized IgG1 monoclonal antibody that is degraded by the proteolytic enzymes widely distributed in the body and not restricted to hepatic tissue.
Specific Populations
Racial Groups and Male and Female Patients: Population pharmacokinetics analyses indicated there was no significant effect of race and gender on mepolizumab clearance.
Age: Population pharmacokinetics analyses indicated there was no significant effect of age on mepolizumab clearance.
Pediatric Patients: Mepolizumab pharmacokinetics following subcutaneous administration in subjects aged 6 to 11 years with severe asthma was investigated in the initial 12-week treatment phase of an open-label clinical trial. Exposures (AUC) following subcutaneous administration of either 40 mg (for children weighing <40 kg) or 100 mg (for children weighing ≥40 kg) were 1.32 and 1.97 times higher, respectively, compared with that observed in adults and adolescents receiving 100 mg. Based on these results, simulation of a 40-mg subcutaneous dose every 4 weeks in children aged 6 to 11 years, irrespective of body weight, resulted in predicted exposures similar to that observed in adults and adolescents.
Patients with Renal Impairment: No clinical trials have been conducted to investigate the effect of renal impairment on the pharmacokinetics of mepolizumab. Based on population pharmacokinetic analyses, mepolizumab clearance was comparable between subjects with creatinine clearance values between 50 and 80 mL/min and patients with normal renal function. There are limited data available in subjects with creatinine clearance values <50 mL/min; however, mepolizumab is not cleared renally.
Patients with Hepatic Impairment: No clinical trials have been conducted to investigate the effect of hepatic impairment on the pharmacokinetics of mepolizumab. Since mepolizumab is degraded by widely distributed proteolytic enzymes, not restricted to hepatic tissue, changes in hepatic function are unlikely to have any effect on the elimination of mepolizumab.
Drug Interaction Studies
No formal drug interaction studies have been conducted with mepolizumab. In population pharmacokinetics analyses of Phase 3 studies, there was no evidence of an effect of commonly coadministered small molecule drugs on mepolizumab exposure.
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of NUCALA or of other mepolizumab products.
In adult and adolescent patients with severe asthma receiving NUCALA 100 mg, 15/260 (6%) had detectable anti-mepolizumab antibodies. Neutralizing antibodies were detected in 1 patient with asthma receiving NUCALA 100 mg. Anti-mepolizumab antibodies slightly increased (approximately 20%) the clearance of mepolizumab. There was no evidence of a correlation between anti-mepolizumab antibody titers and change in eosinophil level. The clinical relevance of the presence of anti-mepolizumab antibodies is not known. In the clinical trial of children aged 6 to 11 years with severe asthma receiving NUCALA 40 or 100 mg, 2/35 (6%) had detectable anti-mepolizumab antibodies during the initial short phase of the trial. No children had detectable anti-mepolizumab antibodies during the long phase of the trial.
In patients receiving NUCALA 100 mg over 52 weeks for CRSwNP or over 52 to 104 weeks for COPD, 6/196 (3%) and 35/999 (4%) had detectable anti-mepolizumab antibodies, respectively. In patients receiving NUCALA 300 mg over 52 weeks for EGPA or over 32 weeks for HES, 1/68 (<2%) and 1/53 (2%) had detectable anti-mepolizumab antibodies, respectively. No neutralizing antibodies were detected in any of these patients. Because of the low occurrence of anti-drug antibodies, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of NUCALA is unknown.
The reported frequency of anti-mepolizumab antibodies may underestimate the actual frequency due to lower assay sensitivity in the presence of high drug concentration. The data reflect the percentage of patients whose test results were positive for antibodies to mepolizumab in specific assays.
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of mepolizumab. Published literature using ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of mepolizumab. Published literature using animal models suggests that IL-5 and eosinophils are part of an early inflammatory reaction at the site of tumorigenesis and can promote tumor rejection. However, other reports indicate that eosinophil infiltration into tumors can promote tumor growth. Therefore, the malignancy risk in humans from an antibody to IL-5 such as mepolizumab is unknown.
Male and female fertility were unaffected based upon no adverse histopathological findings in the reproductive organs from cynomolgus monkeys receiving mepolizumab for 6 months at intravenous dosages up to 100 mg/kg once every 4 weeks (approximately 20 times the MRHD of 300 mg on an AUC basis). Mating and reproductive performance were unaffected in male and female CD-1 mice receiving an analogous antibody, which inhibits the activity of murine IL-5, at an intravenous dosage of 50 mg/kg once per week.
-
14 CLINICAL STUDIES 14.1 Severe Asthma - The asthma development program for NUCALA in patients aged 12 years and older included 3 double-blind, randomized, placebo‑controlled trials: 1 dose-ranging and exacerbation ...
14.1 Severe Asthma
The asthma development program for NUCALA in patients aged 12 years and older included 3 double-blind, randomized, placebo‑controlled trials: 1 dose-ranging and exacerbation trial (DREAM, NCT01000506) and 2 confirmatory trials (MENSA, NCT01691521 and SIRIUS, NCT01691508). Mepolizumab was administered every 4 weeks in all 3 trials as add-on to background treatment. All patients continued their background asthma therapy throughout the duration of the trials.
Dose-Ranging and Exacerbation Trial
DREAM was a 52-week dose-ranging and exacerbation-reduction trial in patients with severe asthma with a history of 2 or more exacerbations in the previous year despite regular use of high‑dose ICS plus additional controller(s) with or without OCS. Patients enrolled in this trial were required to have at least 1 of the following 4 pre-specified criteria in the previous 12 months: blood eosinophil count ≥300 cells/mcL, sputum eosinophil count ≥3%, exhaled nitric oxide concentration ≥50 ppb, or deterioration of asthma control after ≤25% reduction in regular maintenance ICS/OCS. Three intravenous dosages of mepolizumab (75, 250, and 750 mg) administered once every 4 weeks were evaluated compared with placebo. Results from this trial and the pharmacodynamic study supported the evaluation of mepolizumab 75 mg intravenously and 100 mg subcutaneously in the subsequent trials [see Clinical Pharmacology (12.2)]. NUCALA is not indicated for intravenous use and should only be administered by the subcutaneous route.
Confirmatory Trials
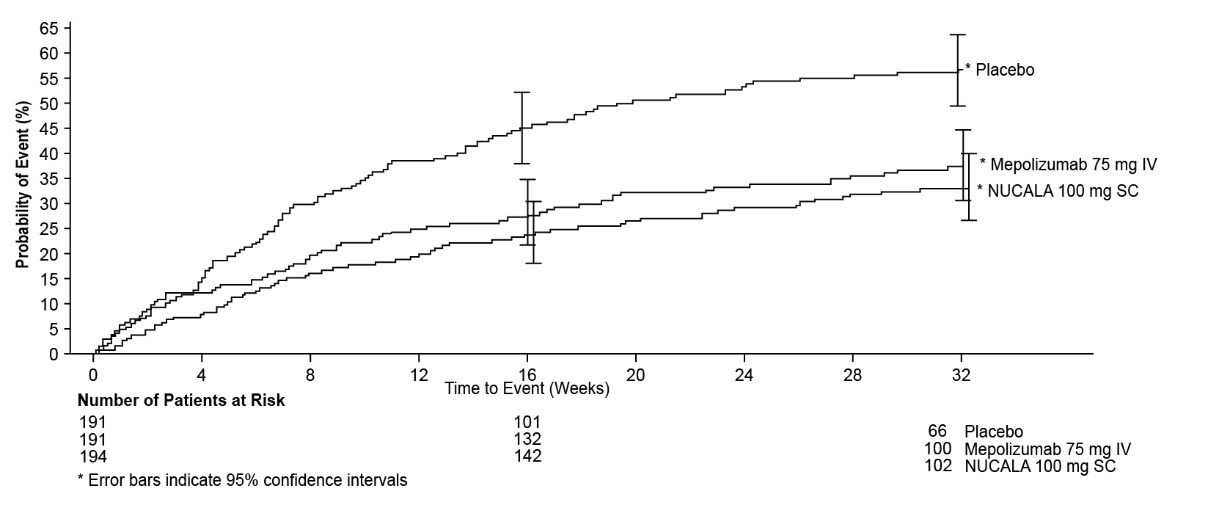
A total of 711 patients with severe asthma were studied in the 2 confirmatory trials (MENSA and SIRIUS). In these 2 trials patients were required to have blood eosinophils of ≥150 cells/mcL at screening (within 6 weeks of dosing) or blood eosinophils of ≥300 cells/mcL within 12 months of enrollment. The screening blood eosinophils of ≥150 cells/mcL criterion was derived from exploratory analyses of data from the DREAM Trial. MENSA was a 32-week placebo‑ and active‑controlled trial in patients with severe asthma with a history of 2 or more exacerbations in the previous year despite regular use of high-dose ICS plus additional controller(s) with or without OCS. Patients received mepolizumab 75 mg intravenously (n = 191), NUCALA 100 mg (n = 194), or placebo (n = 191) once every 4 weeks for 32 weeks.
SIRIUS was a 24-week OCS-reduction trial in patients with severe asthma who required daily OCS in addition to regular use of high-dose ICS plus additional controller(s) to maintain asthma control. Patients in the SIRIUS Trial were not required to have a history of exacerbations in the previous year. Patients received NUCALA 100 mg (n = 69) or placebo (n = 66) once every 4 weeks for 24 weeks. The baseline mean OCS use was similar in the 2 treatment groups: 13.2 mg in the placebo group and 12.4 mg in the group receiving NUCALA 100 mg.
The demographics and baseline characteristics of these 3 trials are provided in Table 5.
Table 5. Demographics and Baseline Characteristics of Severe Asthma Trials FEV1 = forced expiratory volume in 1 second, SABA = short-acting beta2-agonist, FVC = forced vital capacity. DREAM
(N = 616)
MENSA
(N = 576)
SIRIUS
(N = 135)
Mean age, years
49
50
50
Female, n (%)
387 (63)
328 (57)
74 (55)
White, n (%)
554 (90)
450 (78)
128 (95)
Duration of asthma, years, mean
19
20
19
Never smoked, n (%)
483 (78)
417 (72)
82 (61)
Baseline FEV1, L
1.88
1.82
1.95
Baseline % predicted FEV1
60
61
59
Baseline % reversibility
25
27
26
Baseline post-SABA FEV1/FVC
0.67
0.66
0.66
Geometric mean eosinophil count at baseline, cells/mcL
250
290
240
Mean number of exacerbations in previous year
3.6
3.6
3.1
Exacerbations
Efficacy was assessed in DREAM and MENSA using an endpoint of the frequency of exacerbations defined as worsening of asthma requiring use of oral/systemic corticosteroids and/or hospitalization and/or emergency department visits. For patients on maintenance OCS, an exacerbation requiring OCS was defined as the use of oral/systemic corticosteroids at least double the existing dose for at least 3 days. Compared with placebo, patients receiving NUCALA 100 mg or mepolizumab 75 mg intravenously experienced significantly fewer exacerbations. Additionally, compared with placebo, there were fewer exacerbations requiring hospitalization and/or emergency department visits and exacerbations requiring only in-patient hospitalization with NUCALA 100 mg (Table 6).
Table 6. Rate of Exacerbations in Severe Asthma Trials DREAM and MENSA (Intent-to-Treat Population) CI = confidence interval, IV = intravenous, SC = subcutaneous. Trial
Treatment
Exacerbations per Year
Rate
Difference
Rate Ratio
(95% CI)
All exacerbations
DREAM
Placebo (n = 155)
2.40
Mepolizumab 75 mg IV (n = 153)
1.24
1.16
0.52
(0.39, 0.69)
MENSA
Placebo (n = 191)
1.74
Mepolizumab 75 mg IV (n = 191)
0.93
0.81
0.53
(0.40, 0.72)
NUCALA 100 mg SC (n = 194)
0.83
0.91
0.47
(0.35, 0.64)
Exacerbations requiring hospitalization/emergency room visit
DREAM
Placebo (n = 155)
0.43
Mepolizumab 75 mg IV (n = 153)
0.17
0.26
0.40
(0.19, 0.81)
MENSA
Placebo (n = 191)
0.20
Mepolizumab 75 mg IV (n = 191)
0.14
0.06
0.68
(0.33, 1.41)
NUCALA 100 mg SC (n = 194)
0.08
0.12
0.39
(0.18, 0.83)
Exacerbations requiring hospitalization
DREAM
Placebo (n = 155)
0.18
Mepolizumab 75 mg IV (n = 153)
0.11
0.07
0.61
(0.28, 1.33)
MENSA
Placebo (n = 191)
0.10
Mepolizumab 75 mg IV (n = 191)
0.06
0.04
0.61
(0.23, 1.66)
NUCALA 100 mg SC (n = 194)
0.03
0.07
0.31
(0.11, 0.91)
The time to first exacerbation was longer for the groups receiving NUCALA 100 mg and mepolizumab 75 mg intravenously compared with placebo in MENSA (Figure 1).
Figure 1. Kaplan-Meier Cumulative Incidence Curve for Time to First Exacerbation (MENSA)
IV = intravenous, SC = subcutaneous.
DREAM data were explored to determine criteria that could identify patients likely to benefit from treatment with NUCALA. The exploratory analysis suggested that baseline blood eosinophil count of ≥150 cells/mcL was a potential predictor of treatment benefit. Exploratory analysis of MENSA data also suggested that baseline blood eosinophil count (obtained within 6 weeks of initiation of dosing) of ≥150 cells/mcL was a potential predictor of efficacy and showed a trend of greater exacerbation benefit with increasing blood eosinophil count. In MENSA, patients enrolled solely on the basis of the historical blood eosinophil count of ≥300 cells/mcL in the previous 12 months, but who had a baseline blood eosinophil count <150 cells/mcL, had virtually no exacerbation benefit following treatment with NUCALA 100 mg compared with placebo.
The Asthma Control Questionnaire-5 (ACQ-5) was assessed in Trials DREAM and SIRIUS, and the St. George’s Respiratory Questionnaire (SGRQ) was assessed in Trial 2. In Trial 1, the ACQ-5 responder rate (defined as a decrease in score of 0.5 or more as threshold) for the 75-mg intravenous mepolizumab arm was 47% compared with 50% for placebo with an odds ratio (OR) of 1.1 (95% confidence interval [CI]: 0.7, 1.7). In MENSA, the ACQ-5 responder rate for the treatment arm for NUCALA 100 mg was 57% compared with 45% for placebo with an OR of 1.8 (95% CI: 1.2, 2.8). In MENSA, the SGRQ responder rate (defined as a decrease in score of 4 or more as threshold) for the treatment arm for NUCALA 100 mg was 71% compared with 55% for placebo with an OR of 2.1 (95% CI: 1.3, 3.2).
Oral Corticosteroid Reduction
Severe Asthma Trial SIRIUS evaluated the effect of NUCALA 100 mg on reducing the use of maintenance OCS. Efficacy was assessed using an endpoint of the percent reduction of OCS dose during Weeks 20 to 24 compared with baseline dose, while maintaining asthma control. Patients were classified according to their change in OCS use during the trial with the following categories: 90% to 100% decrease, 75% to <90% decrease, 50% to <75% decrease, >0% to <50% decrease, and no improvement (i.e., no change or any increase or lack of asthma control or withdrawal of treatment). Compared with placebo, patients receiving NUCALA 100 mg achieved greater reductions in daily maintenance OCS dose, while maintaining asthma control. Sixteen (23%) patients in the group receiving NUCALA 100 mg versus 7 (11%) in the placebo group had a 90% to 100% reduction in their OCS dose. Twenty-five (36%) patients in the group receiving NUCALA 100 mg versus 37 (56%) in the placebo group were classified as having no improvement for OCS dose. Additionally, 54% of patients receiving NUCALA 100 mg achieved at least a 50% reduction in the daily prednisone dose compared with 33% of patients receiving placebo (95% CI for difference: 4%, 37%). An exploratory analysis was also performed on the subgroup of 29 patients in Trial 3 who had an average baseline and screening blood eosinophil count <150 cells/mcL. Five (29%) patients in the group receiving NUCALA 100 mg versus 0 (0%) in the placebo group had a 90% to 100% reduction in their dose. Four (24%) patients in the group receiving NUCALA 100 mg versus 8 (67%) in the placebo group were classified as having no improvement for OCS dose. The ACQ and SGRQ were also assessed in SIRIUS and showed results similar to those in MENSA.
Lung Function
Change from baseline in mean forced expiratory volume in 1 second (FEV1) was measured in all 3 trials and is presented in Table 7. Compared with placebo, NUCALA 100 mg did not provide consistent improvements in mean change from baseline in FEV1.
Table 7. Change from Baseline in FEV1 (mL) in Severe Asthma Trials FEV1 = forced expiratory volume in 1 second, CI = confidence interval. a Dose = 75 mg intravenous. b FEV1 at Week 52. c Dose = 100 mg subcutaneous. d FEV1 at Week 32. Trial
Difference from Placebo in Mean Change from Baseline FEV1 (mL) (95% CI)
Week 12
Week 24
Weeks 32/52
DREAMa
10 (-87, 108)
5 (-98, 108)
61 (-39, 161)b
MENSAc
52 (-30, 134)
76 (-6, 159)
98 (11, 184)d
SIRIUSc
56 (-91, 203)
114 (-42, 271)
NA
The effect of mepolizumab on lung function was also studied in a 12-week placebo-controlled trial enrolling patients with asthma on a moderate dose of ICS with evidence of symptoms and lung function impairment. Enrollment was not dependent on a history of exacerbations or a pre-specified eosinophil count. Change from baseline in FEV1 at Week 12 was numerically lower in the mepolizumab treatment groups than the placebo group.
14.2 Chronic Rhinosinusitis with Nasal Polyps
A total of 407 adult patients with CRSwNP were evaluated in a randomized, double‑blind, placebo-controlled, multicenter, 52-week trial (NCT03085797). Patients received NUCALA 100 mg or placebo administered subcutaneously once every 4 weeks while continuing nasal corticosteroid therapy. Patients must have received background nasal corticosteroid for ≥8 weeks pre‑screening. Patients had recurrent and symptomatic CRSwNP, and had at least 1 surgery for the removal of nasal polyps within the previous 10 years. Patients were required to have nasal obstruction symptoms with a visual analog scale (VAS) score of >5 out of a maximum score of 10. Patients were also required to have an endoscopic bilateral nasal polyp score (NPS) of ≥5 out of 8 with NPS ≥2 in each nasal cavity. Patients reported nasal obstruction VAS scores daily by placing a single mark on a continuous line labeled from 0 (none) to 100 (as bad as you can imagine). The distance along the line was converted to a 0 to 10 point scale for scoring. For NPS, polyps on each side of the nose were graded on a categorical scale (0 = no polyps, 1 = small polyps in the middle meatus not reaching below the inferior border of the middle concha, 2 = polyps reaching below the lower border of the middle turbinate, 3 = large polyps reaching the lower border of the inferior turbinate or polyps medial to the middle concha, 4 = large polyps causing almost complete congestion/obstruction of the inferior meatus) for a total score of 0 to 8. Sinus CT scans were not performed at baseline nor during treatment to evaluate for sinus opacification.
The co-primary endpoints were change from baseline to Week 52 in total endoscopic NPS (0 to 8 scale) as graded by independent blinded assessors and change from baseline in nasal obstruction VAS score (0 to 10 scale) during Weeks 49 to 52. The key secondary endpoint was the time to first nasal surgery (nasal polypectomy) up to Week 52 in this trial. Other secondary endpoints were change from baseline in loss of smell VAS score during Weeks 49 to 52, and proportion of patients requiring systemic steroids for nasal polyps up to Week 52. All VAS scores were collected daily by the patients and reported on a 0 to 10 scale (0 = none, 10 = as bad as you can imagine).
The demographics and baseline characteristics of patients in this trial are provided in Table 8.
Table 8. Demographics and Baseline Characteristics in Chronic Rhinosinusitis with Nasal Polyps CRSwNP = chronic rhinosinusitis with nasal polyps, SD = standard deviation, OCS = oral corticosteroid, NPS = nasal polyp score, VAS = visual analog scale, CI = confidence interval, AERD = aspirin-exacerbated respiratory disease.
a As graded by independent blinded assessors.N = 407
Mean age, years
49
Female, n (%)
143 (35)
White, n (%)
379 (93)
Mean CRSwNP duration in years (SD)
11.4 (8.4)
Patients with ≥1 surgery in past 10 years (%)
407 (100)
Patients with ≥3 surgeries in past 10 years (%)
124 (30)
OCS use (≥1 course) in past 12 months, n (%)
197 (48)
Mean bilateral endoscopic NPSa, (SD), range 0-8
5.5 (1.29)
Mean nasal obstruction VAS score, (SD), range 0-10
9.0 (0.83)
Geometric mean blood eosinophil cells/mcL (95% CI)
390 (360, 420)
Asthma, n (%)
289 (71)
AERD, n (%)
108 (27)
Endoscopic Nasal Polyp Score and Nasal Obstruction Visual Analog Scale Scores
Patients who received NUCALA 100 mg had a statistically significant improvement (decrease) in bilateral NPS at Week 52 and nasal obstruction VAS score from Weeks 49 to 52 at the end of the 52 week treatment period (Table 9).
Table 9. Analyses of Endpoints in Chronic Rhinosinusitis with Nasal Polyps SD = standard deviation, SE = standard error, CI = confidence interval, NPS = nasal polyp score at Week 52. a Patients with nasal surgery were assigned worst possible score for the period after nasal surgery. Missing data were imputed based on available off-treatment data across treatment arms. Imputations were made stepwise by visit and conditioned on data from previous visits with the same covariates used in the analysis model. b Least square means from analysis using mixed model repeated measures with covariates of treatment group, geographic region, baseline score, and log(e) baseline blood eosinophil count, visit, interaction terms for visit by baseline and visit by treatment. Scoresa
(range)
Placebo
n = 201
NUCALA 100 mg
n = 206
Mean Difference vs. Placebo
(95% CI)Baseline Mean
(SD)
Mean Changeb
(SE)
Baseline Mean
(SD)
Mean Changeb
(SE)
NPS
(0-8)
5.6
(1.41)
0.06
(0.14)
5.4
(1.17)
-0.87 (0.14)
-0.93
(-1.31, -0.55)
Nasal obstruction
(0-10)
9.02
(0.83)
-2.54
(0.25)
8.92
(0.83)
-4.40 (0.25)
-1.86
(-2.53, -1.19)
Nasal Polypectomy
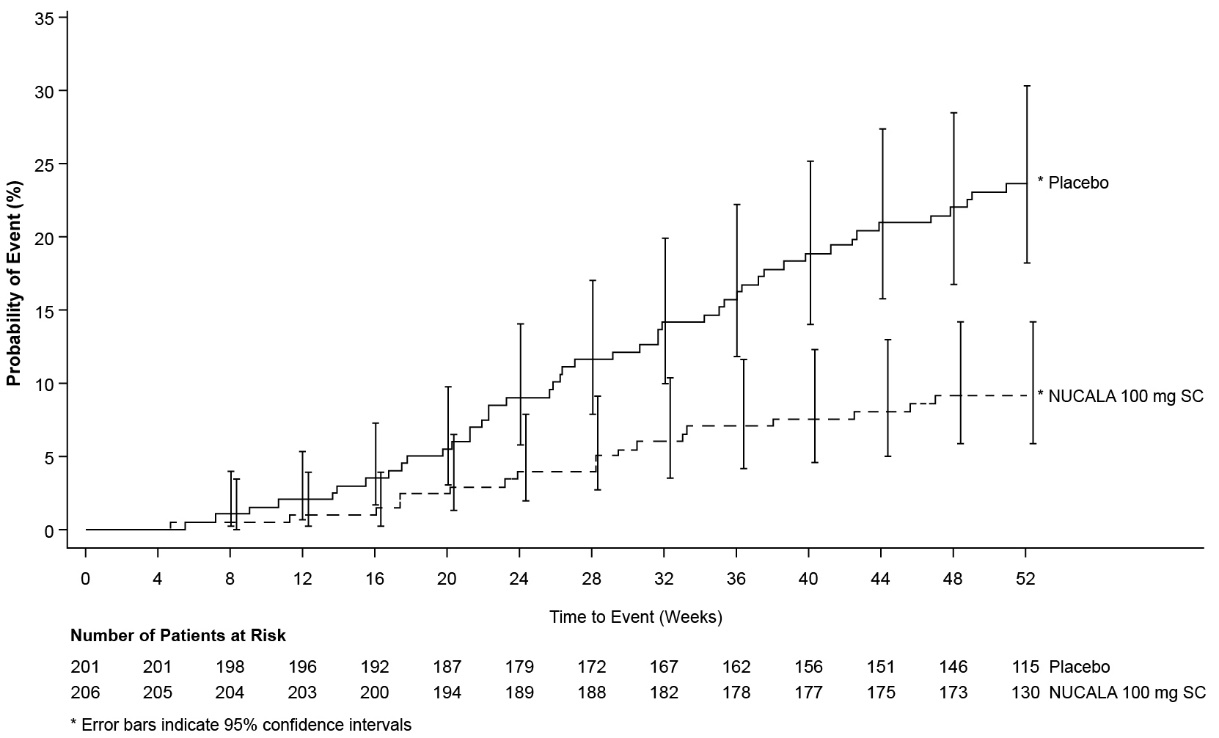
The key secondary endpoint was the time to first nasal surgery (nasal polypectomy) up to Week 52. The proportion of patients who had surgery was significantly reduced by 57% (hazard ratio [HR]: 0.43, 95% CI: 0.25, 0.76) in the group treated with NUCALA 100 mg compared with the placebo group (Figure 2). By Week 52, 18 (9%) patients who received NUCALA 100 mg had surgery compared with 46 (23%) patients in the placebo group.
Figure 2. Kaplan-Meier Plot of Time to First Nasal Surgery in Chronic Rhinosinusitis with Nasal Polyps
SC = subcutaneous.
Additional Chronic Rhinosinusitis with Nasal Polyps Symptoms Scores
For patients who received NUCALA 100 mg, statistically significant improvement was observed in loss of smell compared to placebo and improvements were also observed in the individual VAS symptom scores compared with patients in the placebo group in the 4-weeks prior to the end of the 52-week treatment period (Table 10).
Table 10: Additional Visual Analog Scale Symptom Scores Assessed at Weeks 49-52 VAS = visual analog scale, SD = standard deviation, SE = standard error, CI = confidence interval. a Patients with nasal surgery were assigned the worst possible score for the period after nasal surgery. Missing data was imputed based on available off-treatment data across treatment arms. Imputations were made stepwise by visit and conditioned on data from previous visits with the same covariates used in the analysis model. b Least square means from an analysis using mixed model repeated measures with covariates of treatment group, geographic region, baseline score, and log(e) baseline blood eosinophil count visit, interaction terms for visit by baseline and visit by treatment. c This endpoint was not prespecified in the analysis plan to adjust for multiplicity. VAS Scoresa
(range)
Placebo
n = 201
NUCALA 100 mg
n = 206
Mean Difference vs. Placebo
(95% CI)
Baseline Mean
(SD)
Mean Changeb
(SE)
Baseline Mean
(SD)
Mean Changeb
(SE)
Loss of smell
(0-10)
9.68 (0.60)
-1.46 (0.24)
9.63 (0.83)
-2.92 (0.24)
-1.46
(-2.11, -0.81)
Nasal dischargec
(0-10)
8.78 (1.25)
-2.49 (0.26)
8.78 (1.07)
-4.38 (0.25)
-1.89
(-2.58, -1.20)
Mucus in the throatc
(0-10)
8.58 (1.63)
-2.37 (0.26)
8.51 (1.61)
-4.07 (0.26)
-1.70
(-2.41, -0.99)
Facial painc
(0-10)
7.77 (2.72)
-2.04 (0.28)
7.76 (2.51)
-3.73 (0.27)
-1.69
(-2.43, -0.95)
Corticosteroid Reduction
Treatment with NUCALA 100 mg significantly reduced the need for systemic steroids for nasal polyps vs. placebo up to Week 52 (OR: 0.58, 95% CI: 0.36, 0.92). In patients who received NUCALA 100 mg, 52 (25%) required ≥1 course of systemic steroids compared with 74 (37%) in the placebo group throughout the 52-week treatment period.
Results in Patients with Co-Morbid Asthma
In 289 (71%) patients with co-morbid asthma, pre-specified analyses showed improvements in the co-primary endpoints consistent with those seen in the overall population in the patients who received NUCALA 100 mg compared with placebo. Additionally, based on a post-hoc analysis in these patients, there was a greater response from baseline at Week 52 in asthma control as measured by the ACQ‑5 for NUCALA 100 mg compared with placebo (57% of the NUCALA patients met the responder threshold reduction of ≥0.5, compared to 35% in the placebo group, with an OR of 2.42 [95% CI 1.43, 4.11]).
14.3 Chronic Obstructive Pulmonary Disease
The efficacy of NUCALA as add-on maintenance treatment for adult patients with inadequately controlled chronic obstructive pulmonary disease (COPD) and an eosinophilic phenotype was evaluated in two randomized, double-blind, placebo-controlled, multicenter trials (MATINEE [NCT04133909] and METREX [NCT02105948]). The two trials enrolled a total of 1640 adults who were randomized to receive NUCALA 100 mg or placebo administered subcutaneously every 4 weeks for a treatment duration of 52 to 104 weeks in MATINEE or 52 weeks in METREX. While 1640 adults were enrolled in the two clinical trials (MATINEE and METREX), the efficacy population consisted of 1266 adults.
Both trials enrolled patients with a diagnosis of COPD with moderate to very severe airflow limitation (post-bronchodilator FEV1/FVC ratio <0.7 and post-bronchodilator FEV1 of 20% to 80% predicted) and at least 2 moderate or 1 severe COPD exacerbation in the previous year despite receiving triple inhaled therapy.
In MATINEE, patients were required to have a minimum blood eosinophil count of 300 cell/mcL at screening. In METREX, there was no minimum blood eosinophil count requirement, but randomization was stratified by baseline blood eosinophil count: ≥150 cell/mcL at screening or ≥300 cell/mcL in the previous 12 months, or blood eosinophil count <150 cells/mcL at screening with no evidence of blood eosinophil count ≥300 cell/mcL in the previous 12 months. There was insufficient data from METREX to support the efficacy of NUCALA in patients with COPD with blood eosinophil count <150 cells/mcL at screening with no evidence of blood eosinophil count ≥300 cell/mcL in the previous 12 months. Thus, the efficacy population (N = 1266) included patients from MATINEE (n = 804) and patients from METREX who had a blood eosinophil count ≥150 cell/mcL at screening or ≥300 cell/mcL in the previous 12 months (n = 462). The data from this efficacy population is described below.
The demographic and baseline characteristics of MATINEE and METREX efficacy population are provided in Table 11 below.
Table 11: Demographics and Baseline Characteristics of Patients with Chronic Obstructive Pulmonary Disease in MATINEE and METREXa Trials SD = standard deviation, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, COPD = chronic obstructive pulmonary disease, ICS = inhaled corticosteroids, LAMA = long-acting muscarinic antagonist, LABA = long-acting beta agonist, SGRQ = St. George’s Respiratory Questionnaire, CI = confidence interval. a Patients with blood eosinophil count ≥150 cell/mcL at screening or ≥300 cell/mcL in the previous 12 months only. b Exacerbations treated with either systemic corticosteroids with or without antibiotics. c Exacerbations requiring hospitalization. MATINEE
METREXa
N = 804
N = 462
Mean age (y) (SD)
66 (8.0)
65 (8.4)
Female, n (%)
253 (31)
163 (35)
White, n (%)
673 (84)
391 (85)
Asian, n (%)
112 (14)
5 (1)
Black or African American, n (%)
10 (1)
6 (1)
Other/Multiple, n (%)
9 (1)
60 (13)
Hispanic/Latino, n (%)
189 (24)
75 (16)
Current smokers, n (%)
222 (28)
134 (29)
Average smoking history (pack-years) (SD)
43 (24.9)
44 (25.8)
Post-bronchodilator % predicted FEV1, mean (SD)
48 (15.8)
44 (14.8)
Post-bronchodilator FEV1/FVC, mean (SD)
0.5 (0.12)
0.5 (0.12)
Mean number of moderateb or severec exacerbations in previous year (SD)
2.3 (0.94)
2.5 (1.29)
Background COPD medications at randomization:
ICS/LAMA/LABA, n (%)
794 (99)
454 (98)
SGRQ score, mean (SD)
55 (17.8)
55 (16.7)
Geometric mean eosinophil count at screening, cells/mcL (95% CI)
480 (470, 490)
260 (250, 280)
Annualized Rate of Moderate or Severe Exacerbations in Adult Patients with Chronic Obstructive Pulmonary Disease
The primary endpoint for the MATINEE and METREX trials was the annualized rate of moderate or severe exacerbations during the 52 to 104-week and 52-week treatment periods, respectively. Moderate exacerbations are defined per protocol as clinically significant exacerbations that require treatment with oral/systemic corticosteroids and/or antibiotics. Severe exacerbations are defined per protocol as clinically significant exacerbations that require in‑patient hospitalization (i.e., ≥24 hours) or result in death.
In both trials, NUCALA demonstrated a statistically significant reduction in the annualized rate of moderate or severe exacerbations compared with placebo when added to triple inhaled therapy (see Table 12).
Table 12. Annualized Rate of Moderatea or Severeb Exacerbations in MATINEE and METREX Trials CI = confidence interval. a Exacerbations treated with either systemic corticosteroids/antibiotics. b Exacerbations requiring hospitalization or resulting in death. c Patients with baseline eosinophils >150 cell/mcL at screening or ≥300 cell/mcL in the previous 12 months. MATINEE
METREXc
NUCALA
N = 403
Placebo
N = 401
NUCALA
N = 233
Placebo
N = 229
Exacerbation rate per year
0.80
1.01
1.40
1.71
Rate ratio vs. placebo
(95% CI)
0.79
(0.66, 0.94)
0.82
(0.68, 0.98)
The time to first event analysis showed a statistically significant reduction in the risk of moderate or severe exacerbation for patients receiving NUCALA compared to placebo (HR: 0.77; 95% CI: 0.64, 0.93) through 104 weeks in MATINEE.
NUCALA reduced the annualized rate of COPD exacerbations requiring emergency department visits and/or hospitalization when compared with placebo (rate ratio [RR] of 0.65; 95% CI: 0.43, 0.96 [not statistically significant due to failure of an endpoint higher in the pre-defined testing hierarchy]) in MATINEE.
Health Related Quality of Life
In MATINEE and METREX, the St. George’s Respiratory Questionnaire (SGRQ) total score responder rate (defined as the proportion of subjects with SGRQ improvement from baseline of at least 4 points) at Week 52 was evaluated. In MATINEE, the responder rate was 50% in the NUCALA group compared with 46% in the placebo group (N = 783, odds ratio [OR]: 1.17; 95% CI: 0.87, 1.57). In the METREX efficacy population, the responder rate was 42% in the NUCALA group compared to 40% in the placebo group (N = 451, odds ratio [OR]: 1.08; 95% CI: 0.74, 1.59).
14.4 Eosinophilic Granulomatosis with Polyangiitis
A total of 136 adult patients with EGPA were evaluated in a randomized, placebo-controlled, multicenter, 52-week trial (NCT02020889). Patients received 300 mg of NUCALA or placebo administered subcutaneously once every 4 weeks while continuing their stable OCS therapy. Starting at Week 4, OCS was tapered during the treatment period at the discretion of the investigator. Efficacy was assessed in this trial using co-endpoints of the total accrued duration of remission over the 52-week treatment period, defined as Birmingham Vasculitis Activity Score (BVAS) = 0 (no active vasculitis) plus prednisolone or prednisone dose less than or equal to 4 mg/day, and the proportion of patients in remission at both Week 36 and Week 48 of treatment. The BVAS is a clinician-completed tool to assess clinically active vasculitis that would likely require treatment, after exclusion of other causes.
The demographics and baseline characteristics of patients in this trial are provided in Table 13.
Table 13. Demographics and Baseline Characteristics in Eosinophilic Granulomatosis with Polyangiitis EGPA = eosinophilic granulomatosis with polyangiitis, SD = standard deviation. a Prednisone or prednisolone equivalent. b e.g., azathioprine, methotrexate, mycophenolic acid. N = 136
Mean age, years
48.5
Female, n (%)
80 (59)
White, n (%)
125 (92)
Duration of EGPA, years, mean (SD)
5.5 (4.63)
History of >1 confirmed relapse in past 2 years, n (%)
100 (74)
Refractory disease, n (%)
74 (54)
Recurrence of EGPA symptoms, n (%)
68 (50)
Failed induction treatment, n (%)
6 (4)
Baseline oral corticosteroida daily dose, mg, median (range)
12 (7.5-50)
Receiving immunosuppressive therapy,b n (%)
72 (53)
Remission
Patients receiving 300 mg of NUCALA achieved a significantly greater accrued time in remission compared with placebo. A significantly higher proportion of patients receiving 300 mg of NUCALA achieved remission at both Week 36 and Week 48 compared with placebo (Table 14). Results of the components of remission are also shown in Table 14. In addition, significantly more patients receiving 300 mg of NUCALA achieved remission within the first 24 weeks and remained in remission for the remainder of the 52-week trial treatment period compared with placebo (19% for 300 mg of NUCALA versus 1% for placebo; OR: 19.7; 95% CI: 2.3, 167.9).
Table 14. Remission and Components of Remission in Eosinophilic Granulomatosis with Polyangiitis OCS = oral corticosteroid, BVAS = Birmingham Vasculitis Activity Score, CI = confidence interval.
a An odds ratio >1 favors NUCALA.Remission
(OCS ≤4 mg/day + BVAS = 0)
OCS ≤4 mg/day
BVAS = 0
Placebo
n = 68
NUCALA
300 mg
n = 68
Placebo
n = 68
NUCALA
300 mg
n = 68
Placebo
n = 68
NUCALA
300 mg
n = 68
Accrued duration over 52 weeks, n (%)
0
55 (81)
32 (47)
46 (68)
27 (40)
6 (9)
3 (4)
>0 to <12 weeks
8 (12)
8 (12)
12 (18)
5 (7)
15 (22)
13 (19)
12 to <24 weeks
3 (4)
9 (13)
6 (9)
12 (18)
11 (16)
5 (7)
24 to <36 weeks
0
10 (15)
2 (3)
10 (15)
17 (25)
2 (3)
≥36 weeks
2 (3)
9 (13)
2 (3)
14 (21)
19 (28)
45 (66)
Odds ratio (NUCALA/placebo)a
5.9
5.1
3.7
(95% CI)
(2.7, 13.0)
(2.5, 10.4)
(1.8, 7.6)
Proportion of patients at both Weeks 36 and 48
Patients, n (%)
2 (3)
22 (32)
7 (10)
28 (41)
23 (34)
34 (50)
Odds ratio (NUCALA/placebo)a
16.7
6.6
1.9
(95% CI)
(3.6, 77.6)
(2.6, 17.1)
(0.9, 4.2)
Additionally, a statistically significant benefit for these endpoints was demonstrated using remission defined as BVAS = 0 plus prednisolone/prednisone ≤7.5 mg/day.
Relapse
The time to first relapse (defined as worsening related to vasculitis, asthma, or sino-nasal symptoms requiring an increase in dose of corticosteroids or immunosuppressive therapy or hospitalization) was significantly longer for patients receiving 300 mg of NUCALA compared with placebo with an HR of 0.32 (95% CI: 0.21, 0.5) (Figure 3). Additionally, patients receiving 300 mg of NUCALA had a reduction in rate of relapse compared with patients receiving placebo (RR: 0.50; 95% CI: 0.36, 0.70 for 300 mg of NUCALA compared with placebo). The incidence and number of relapse types (vasculitis, asthma, sino-nasal) were numerically lower with NUCALA compared with placebo.
Figure 3. Kaplan-Meier Plot of Time to First Relapse in Eosinophilic Granulomatosis with Polyangiitis
SC = subcutaneous.
Corticosteroid Reduction
Patients receiving 300 mg of NUCALA had a significantly greater reduction in average daily OCS dose compared with patients receiving placebo during Weeks 48 to 52 (Table 15).
Table 15. Average Daily Oral Corticosteroid Dose during Weeks 48 to 52 in Eosinophilic Granulomatosis with Polyangiitis CI = confidence interval.
a Analyzed using a proportional odds model with covariates of treatment group, baseline oral corticosteroid daily dose, baseline Birmingham Vasculitis Activity Score, and region.
b An odds ratio <1 favors NUCALA.Number (%) of Patients
Placebo
n = 68
NUCALA
300 mg
n = 68
0
2 (3)
12 (18)
>0 to ≤4.0 mg
3 (4)
18 (26)
>4.0 to ≤7.5 mg
18 (26)
10 (15)
>7.5 mg
45 (66)
28 (41)
Comparison: NUCALA/placeboa
Odds ratiob
0.20
95% CI
0.09, 0.41
Asthma Control Questionnaire-6 (ACQ-6)
The ACQ-6, a 6-item questionnaire completed by the patient, was developed to measure the adequacy of asthma control and change in asthma control. The on-treatment ACQ-6 responder rate during Weeks 48 to 52 (defined as a decrease in score of 0.5 or more compared with baseline) was 22% for 300 mg of NUCALA and 16% for placebo (OR: 1.56; 95% CI: 0.63, 3.88 for 300 mg of NUCALA compared with placebo).
Close14.5 Hypereosinophilic Syndrome
A total of 108 adult and adolescent patients aged 12 years and older with HES for at least 6 months were evaluated in a randomized, double-blind, placebo-controlled, multicenter, 32‑week trial (NCT02836496). Patients with non-hematologic secondary HES (e.g., drug hypersensitivity, parasitic helminth infection, HIV infection, non-hematologic malignancy) or FIP1L1-PDGFRα kinase-positive HES were excluded from the trial. Patients received 300 mg of NUCALA or placebo subcutaneous once every 4 weeks while continuing their stable HES therapy. Patients entering the trial had experienced at least 2 HES flares within the past 12 months and a blood eosinophil count of 1,000 cells/mcL or higher during screening. Historical HES flares for the trial entry criteria were defined as HES‑related worsening of clinical symptoms or blood eosinophil counts requiring an escalation in therapy. Patients must have been on stable HES therapy for the 4 weeks prior to randomization. HES therapy could include chronic or episodic OCS, immunosuppressive, or cytotoxic therapy.
The efficacy of NUCALA in HES was established based upon the proportion of patients who experienced a HES flare during the 32-week treatment period. A HES flare was defined as worsening of clinical signs and symptoms of HES or increasing eosinophils (on at least 2 occasions), resulting in the need to increase OCS or increase/add cytotoxic or immunosuppressive HES therapy.
The demographics and baseline characteristics of patients in this trial are provided in Table 16.
Table 16. Demographics and Baseline Characteristics in Hypereosinophilic Syndrome SD = standard deviation, HES = Hypereosinophilic Syndrome. N = 108
Mean age, years (SD)
46.0 (15.78)
Female, n (%)
57 (53)
White, n (%)
100 (93)
Mean duration of HES, years
5.55
Flares
The trial compared the proportion of patients who experienced a HES flare or withdrew from the trial in the NUCALA and placebo treatment groups (Table 17). Over the 32-week treatment period, the incidence of HES flare over the treatment period was 56% for the placebo group and 28% for the group treated with NUCALA (50% reduction).
Table 17. Overview of Hypereosinophilic Syndrome Flares HES = Hypereosinophilic Syndrome, CMH = Cochran-Mantel-Haenszel, CI = confidence interval.
a Analysis compared the number of patients who experienced ≥1 HES flare and/or withdrew from the trial prematurely.
b An odds ratio <1 favors NUCALA.Number (%) of Patients
Placebo
n = 54
NUCALA
300 mg
n = 54
Patients with ≥1 HES flare or who withdrew from trial
30 (56)
15 (28)
Patients with ≥1 HES flare
28 (52)
14 (26)
Patients with no HES flare who withdrew from trial
2 (4)
1 (2)
Comparison: NUCALA/placeboa
CMH P value
0.002
Odds ratiob
0.28
95% CI
(0.12, 0.64)
Time to First Flare
Difference was observed between NUCALA and placebo arms in the time to first HES flare (Figure 4). The risk of first HES flare over the treatment period was 66% lower for patients treated with NUCALA compared with placebo (HR: 0.34; 95% CI 0.18, 0.67, P = 0.002).
Figure 4. Kaplan-Meier Curve for Time to First Hypereosinophilic Syndrome Flare
SC = subcutaneous.
Proportion of Patients Who Experienced Flares during Week 20 through Week 32
From Week 20 through Week 32, significantly fewer patients experienced a HES flare or withdrew from the trial when treated with 300 mg of NUCALA compared with placebo (17% vs. 35%, respectively, P = 0.020; OR: 0.33; 95 % CI: 0.13, 0.85).
Rate of Flares
Patients who received NUCALA experienced significantly fewer HES flares during the 32-week treatment period compared with the placebo group (Table 18). Treatment with NUCALA resulted in a statistically significant 66% reduction in the annualized rate of HES flares compared with placebo.
Table 18. Frequency of Flares CI = confidence interval.a
Adjusted P values based on pre-specified hierarchy of endpoints.
b A rate ratio <1 favors NUCALA.Number (%) of Patients
Placebo
n = 54
NUCALA
300 mg
n = 54
0
26 (48)
40 (74)
1
15 (28)
11 (20)
2
7 (13)
3 (6)
3
5 (9)
0
4
1 (2)
0
≥5
0
0
Comparison: NUCALA/placebo
Wilcoxon P value (unadjusted/adjusted)a
0.002/0.02
Rate/year
1.46
0.50
Rate ratiob
0.34
95% CI
(0.19, 0.63)
Brief Fatigue Inventory
Brief Fatigue Inventory (BFI) Item 3 asks patients to record their worst level of weariness/tiredness severity during the past 24 hours (scale: 0 = no fatigue to 10 = as bad as you can imagine). At baseline, median BFI Item 3 scores were similar between treatment groups (4.46 for NUCALA 300 mg and 4.69 for placebo). At Week 32, BFI Item 3 scores improved with NUCALA compared with placebo (P = 0.036). The median change from baseline score for BFI Item 3 at Week 32 was -0.66 in the group treated with NUCALA and 0.32 in the placebo group.
-
16 HOW SUPPLIED/STORAGE AND HANDLING NUCALA for Injection - NUCALA (mepolizumab) for injection is a sterile, preservative-free, white to off-white, lyophilized powder for reconstitution and subcutaneous injection in a single-dose ...
NUCALA for Injection
NUCALA (mepolizumab) for injection is a sterile, preservative-free, white to off-white, lyophilized powder for reconstitution and subcutaneous injection in a single-dose glass vial with a flip-off seal. The vial stopper is not made with natural rubber latex.
NUCALA for injection is supplied as:
100-mg single-dose vials in cartons of 1 (NDC 0173-0881-01).
Store vials below 77°F (25°C). Do not freeze. Store in the original carton to protect from light.
NUCALA Injection
NUCALA (mepolizumab) injection is a sterile, preservative-free, clear to opalescent, colorless to pale yellow to pale brown solution for subcutaneous use. Each single-dose prefilled autoinjector delivers 100 mg of mepolizumab in 1 mL of solution. Each single-dose prefilled syringe delivers 100 mg of mepolizumab in 1 mL of solution or 40 mg of mepolizumab in 0.4 mL of solution. The autoinjectors and syringes are not made with natural rubber latex.
NUCALA injection is supplied as:
- •
- 100 mg/mL, single-dose, prefilled autoinjector with attached 29-gauge, half-inch needle in cartons of 1 (NDC 0173-0892-01).
- •
- 100 mg/mL, single-dose, prefilled glass syringe with attached 29-gauge, half-inch needle with a needle guard in cartons of 1 (NDC 0173-0892-42).
- •
- 40 mg/0.4 mL, single-dose, prefilled glass syringe with attached 29-gauge, half-inch needle with a needle guard in cartons of 1 (NDC 0173-0904-42).
Prior to Dispensing: Refrigerate prefilled autoinjectors and prefilled syringes at 36°F to 46°F (2°C to 8°C). Keep the product in the original carton to protect from light. Do not freeze. Do not shake. Avoid exposure to heat.
Following Dispensing: Refrigerate prefilled autoinjectors and prefilled syringes at 36°F to 46°F (2°C to 8°C). Keep the product in the original carton to protect from light until the time of use. Do not freeze. Do not shake. Avoid exposure to heat.
If necessary, an unopened carton can be stored outside the refrigerator at up to 86°F (30°C) for up to 7 days. Discard if left out of the refrigerator for more than 7 days.
NUCALA injection must be administered within 8 hours after removal from the carton. Discard if not administered within 8 hours.
Close -
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Hypersensitivity Reactions - Inform patients that hypersensitivity reactions (e.g. ...
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Inform patients that hypersensitivity reactions (e.g., anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, rash) have occurred after administration of NUCALA. Instruct patients to contact their healthcare provider if such reactions occur.
Not for Acute Symptoms or Deteriorating Disease
Inform patients that NUCALA does not treat acute asthma symptoms or acute exacerbations of asthma or COPD. Inform patients to seek medical advice if their asthma remains uncontrolled or COPD symptoms worsen after initiation of treatment with NUCALA.
Opportunistic Infections: Herpes Zoster
Inform patients that herpes zoster infections have occurred in patients receiving NUCALA and where medically appropriate, inform patients that vaccination should be considered.
Reduction of Corticosteroid Dosage
Inform patients to not discontinue systemic or inhaled corticosteroids except under the direct supervision of a physician. Inform patients that reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Trademarks are owned by or licensed to the GSK group of companies.
Manufactured by
GlaxoSmithKline LLC
Philadelphia, PA 19104
U.S. License No. 1727Distributed by
GlaxoSmithKline
Durham, NC 27701©2025 GSK group of companies or its licensor.
NCL:11PI
Close -
PATIENT PACKAGE INSERTPatient Information - NUCALA (new KAH la) (mepolizumab) for injection, for subcutaneous use - NUCALA (new KAH la) (mepolizumab) injection, for subcutaneous use - What is ...
Patient Information
NUCALA (new KAH la)
(mepolizumab)
for injection, for subcutaneous use
NUCALA (new KAH la)
(mepolizumab)
injection, for subcutaneous use
What is NUCALA?
- •
- NUCALA is a prescription medicine:
- o
- for the add-on maintenance treatment of severe asthma in people 6 years of age and older whose asthma is not controlled with their current asthma medicines. NUCALA helps prevent severe asthma attacks (exacerbations).
NUCALA is not used to treat sudden breathing problems that occur with asthma. - o
- for add-on maintenance treatment of chronic rhinosinusitis with nasal polyps (CRSwNP) in adults whose disease is not controlled with nasal corticosteroids. NUCALA helps reduce symptoms (e.g., nasal congestion, nasal discharge, mucus in the throat, loss of smell). NUCALA helps reduce the size of your nasal polyps and the use of oral corticosteroid medicines. NUCALA helps prevent surgery for your nasal polyps.
- o
- for add-on maintenance treatment of chronic obstructive pulmonary disease (COPD) in adults whose disease is not controlled and who are at risk of flare-ups and increasing symptoms. NUCALA helps prevent flare-ups of COPD symptoms (exacerbations).
NUCALA is not used to treat sudden breathing problems that can occur with COPD. - o
- for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA). NUCALA helps reduce symptoms and flares, and it may allow your healthcare provider to reduce your oral corticosteroid medicine.
- o
- for the treatment of people 12 years of age and older with hypereosinophilic syndrome (HES) for 6 months or more. NUCALA helps reduce symptoms and prevent flares.
- •
- NUCALA works by targeting inflammation that plays a major role in severe asthma, CRSwNP, COPD, EGPA and HES. NUCALA reduces blood eosinophils. Eosinophils are a type of white blood cells that may contribute to your disease.
It is not known if NUCALA is safe and effective in:
- •
- children with severe asthma under 6 years of age.
- •
- children and adolescents with CRSwNP, COPD or EGPA under 18 years of age; or
- •
- children with HES under 12 years of age.
Do not use NUCALA if you are allergic to mepolizumab or any of the ingredients in NUCALA. See the end of this leaflet for a complete list of ingredients in NUCALA.
Before receiving NUCALA, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have a parasitic (helminth) infection.
- •
- are taking oral or inhaled corticosteroid medicines. Do not stop taking your corticosteroid medicines unless instructed by your healthcare provider. This may cause other symptoms that were controlled by the corticosteroid medicine to come back.
- •
- are pregnant or plan to become pregnant. It is not known if NUCALA may harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. You and your healthcare provider should decide if you will use NUCALA and breastfeed. You should not do both without talking with your healthcare provider first.
- •
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
- •
- Do not stop taking your other medicines unless instructed to do so by your healthcare provider.
How will I receive NUCALA?
Your healthcare provider will prescribe the dose that is right for you depending on what you are being treated for.
When injection is given by a healthcare provider:
- •
- A healthcare provider will inject NUCALA under your skin (subcutaneously) every 4 weeks.
When injection is given by a patient or patient caregiver with a prefilled syringe or prefilled autoinjector:
- •
- NUCALA may be prescribed as a single-dose prefilled autoinjector for people 12 years of age and older or as a single-dose prefilled syringe for people 6 years of age and older.
- •
- Use NUCALA every 4 weeks exactly as your healthcare provider tells you to.
- •
- Before you use NUCALA, your healthcare provider will show you or your caregiver how to give the injections.
- •
- Read the Instructions for Use that comes with NUCALA for instructions about the right way to give your injections at home.
- •
- You should inject NUCALA under your skin (subcutaneously) into your thigh or stomach (abdomen). Also, a caregiver may give the injection in the upper arm.
- •
- If you miss a dose, inject a dose as soon as possible. Then continue (resume) your injection on your regular dosing schedule. If you do not notice that you have missed a dose until it is time for your next scheduled dose, then inject the next scheduled dose as planned. If you are not sure when to inject NUCALA, call your healthcare provider.
What are the possible side effects of NUCALA?
NUCALA can cause serious side effects, including:
- •
- allergic (hypersensitivity) reactions, including anaphylaxis. Serious allergic reactions can happen after you get your NUCALA injection. Allergic reactions can sometimes happen hours or days after you get a dose of NUCALA. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of an allergic reaction:
- o
- swelling of your face, mouth, and tongue
- o
- fainting, dizziness, feeling lightheaded (low blood pressure)
- o
- hives
- o
- breathing problems
- o
- rash
- •
- herpes zoster infections. Herpes zoster infections that can cause shingles have happened in people who received NUCALA.
The most common side effects of NUCALA include: headache, injection site reactions (pain, redness, swelling, itching, or a burning feeling at the injection site), back pain, and tiredness (fatigue). Mouth/throat pain and joint pain have been reported with CRSwNP. Diarrhea and cough have been reported with COPD.
These are not all of the possible side effects of NUCALA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NUCALA?
- •
- Store prefilled autoinjectors and prefilled syringes in the refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- Keep prefilled autoinjectors and prefilled syringes in the original carton until time of use to protect from light.
- •
- Do not freeze. Do not shake. Keep away from heat.
- •
- If necessary, an unopened carton can be stored outside the refrigerator at up to 86°F (30°C) for up to 7 days.
- •
- Safely throw away prefilled autoinjectors and prefilled syringes if the unopened carton is left out of the refrigerator for more than 7 days.
- •
- Prefilled autoinjectors and prefilled syringes must be used within 8 hours after you take them out of the carton. Safely throw away if not used within 8 hours.
- •
- Safely throw away medicine that is out of date or no longer needed.
Keep NUCALA and all medicines out of the reach of children.
General information about the safe and effective use of NUCALA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not give NUCALA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NUCALA that is written for health professionals.
What are the ingredients in NUCALA?
Active Ingredient: mepolizumab.
Inactive Ingredients (vials): polysorbate 80, sodium phosphate dibasic heptahydrate, and sucrose.
Inactive Ingredients (prefilled autoinjectors and prefilled syringes): citric acid monohydrate, EDTA disodium dihydrate, polysorbate 80, sodium phosphate dibasic heptahydrate, and sucrose.
For more information about NUCALA, call 1-888-825-5249 or visit our website at www.NUCALA.com.
Trademarks are owned by or licensed to the GSK group of companies.
Manufactured by:
GlaxoSmithKline LLC, Philadelphia, PA 19104, U.S. License No. 1727
Distributed by:
GlaxoSmithKline, Durham, NC 27701
©2025 GSK group of companies or its licensor.
NCL:10PIL
- This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: May 2025
-
INSTRUCTIONS FOR USEInstructions for Use - NUCALA - (mepolizumab) injection, for subcutaneous use - 100 mg/mL - Prefilled Autoinjector - Important information - NUCALA is a prescription medicine that ...Close
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 0173-0881-01 - Nucala - (mepolizumab) for Injection - 100 mg/vial - Rx Only - GSK - For subcutaneous injection after reconstitution. Reconstituted solution contains ...
PRINCIPAL DISPLAY PANEL
NDC 0173-0881-01
Nucala
(mepolizumab)
for Injection
100 mg/vial
Rx Only
GSK
For subcutaneous injection after reconstitution.
Reconstituted solution contains 100mg/mL.
Single-dose vial. Discard unused portion.
Contents: Each vial delivers 100 mg of mepolizumab, polysorbate 80 (0.67 mg), sodium phosphate dibasic heptahydrate (7.14 mg), and sucrose (160 mg).
No preservative.
No U.S. standard of potency.
1 vial
Do not accept if glued carton seals are broken.
©2023 GSK group of companies or its licensor.
- Rev. 4/23
- 62000000087874
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 0173-0892-01 - Nucala - (mepolizumab) 100 mg/mL - Rx Only - GSK - Prefilled Autoinjector - For subcutaneous use only. Contents: -1 Single-Dose 1-mL. Prefilled ...
PRINCIPAL DISPLAY PANEL
NDC 0173-0892-01
Nucala
(mepolizumab)
100 mg/mL
Rx Only
GSK
Prefilled Autoinjector
For subcutaneous use only.
Contents:
-1 Single-Dose 1-mL. Prefilled Autoinjector
-Instructions for Use
-Prescribing Information
-Patient Information
©2023 GSK group of companies or its licensor.
Do not accept if security seal is missing or broken.
- 62000000087671 Rev. 5/23
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 0173-0904-42 - Nucala - (mepolizumab) Injection - 40mg/0.4 mL - Rx Only - GSK - Prefilled Syringe - For subcutaneous use only. Contents: - 1 Single-Dose 0.4-mL ...
PRINCIPAL DISPLAY PANEL
NDC 0173-0904-42
Nucala
(mepolizumab)
Injection
40mg/0.4 mL
Rx Only
GSK
Prefilled Syringe
For subcutaneous use only.
Contents:
- 1 Single-Dose 0.4-mL Prefilled Syringe
- Instructions for Use
- Prescribing Information
- Patient Information
©2023 GSK group of companies or its licensor.
Do not accept if security seal is missing or broken.
Rev. 4/23
62000000087781
Close -
INGREDIENTS AND APPEARANCEProduct Information
NUCALA mepolizumab injection, powder, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0173-0881 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEPOLIZUMAB (UNII: 90Z2UF0E52) (MEPOLIZUMAB - UNII:90Z2UF0E52) MEPOLIZUMAB 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0173-0881-01 1 in 1 CARTON 11/04/2015 1 1 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:0173-0881-61 1 in 1 CARTON 06/17/2016 2 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125526 11/04/2015 NUCALA mepolizumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0173-0892 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEPOLIZUMAB (UNII: 90Z2UF0E52) (MEPOLIZUMAB - UNII:90Z2UF0E52) MEPOLIZUMAB 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0173-0892-01 1 in 1 CARTON 06/06/2019 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:0173-0892-42 1 in 1 CARTON 06/06/2019 2 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:0173-0892-61 1 in 1 CARTON 06/06/2019 3 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:0173-0892-63 1 in 1 CARTON 06/06/2019 4 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761122 06/06/2019 NUCALA mepolizumab injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0173-0904 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEPOLIZUMAB (UNII: 90Z2UF0E52) (MEPOLIZUMAB - UNII:90Z2UF0E52) MEPOLIZUMAB 40 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0173-0904-42 1 in 1 CARTON 01/22/2022 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761122 01/22/2022
CloseLabeler - GlaxoSmithKline LLC (167380711)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
NUCALA- mepolizumab injection, powder, for solution
NUCALA- mepolizumab injection, solution
Number of versions: 21
| Published Date (What is this?) | Version | Files |
|---|---|---|
| May 26, 2025 | 22 (current) | download |
| Oct 28, 2024 | 21 | download |
| Nov 1, 2023 | 20 | download |
| Mar 17, 2023 | 19 | download |
| Oct 21, 2022 | 18 | download |
| Mar 17, 2022 | 17 | download |
| Oct 6, 2021 | 15 | download |
| Aug 2, 2021 | 14 | download |
| Jun 2, 2021 | 13 | download |
| Oct 2, 2020 | 12 | download |
| Nov 1, 2019 | 11 | download |
| Sep 16, 2019 | 10 | download |
| Jun 7, 2019 | 9 | download |
| Mar 13, 2019 | 8 | download |
| Nov 14, 2018 | 7 | download |
| Dec 12, 2017 | 6 | download |
| Feb 21, 2017 | 5 | download |
| Nov 7, 2016 | 4 | download |
| Jun 24, 2016 | 3 | download |
| Nov 10, 2015 | 2 | download |
| Nov 5, 2015 | 1 | download |
RxNorm
NUCALA- mepolizumab injection, powder, for solution
NUCALA- mepolizumab injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1720601 | mepolizumab 100 MG Injection | PSN |
| 2 | 1720601 | mepolizumab 100 MG Injection | SCD |
| 3 | 1720606 | Nucala 100 MG Injection | PSN |
| 4 | 1720606 | mepolizumab 100 MG Injection [Nucala] | SBD |
| 5 | 1720606 | Nucala 100 MG Injection | SY |
| 6 | 2170990 | mepolizumab 100 MG in 1 mL Auto-Injector | PSN |
| 7 | 2170990 | 1 ML mepolizumab 100 MG/ML Auto-Injector | SCD |
| 8 | 2170990 | mepolizumab 100 MG per 1 ML Auto-Injector | SY |
| 9 | 2170993 | Nucala 100 MG in 1 mL Auto-Injector | PSN |
| 10 | 2170993 | 1 ML mepolizumab 100 MG/ML Auto-Injector [Nucala] | SBD |
| 11 | 2170993 | 1 ML Nucala 100 MG/ML Auto-Injector | SY |
| 12 | 2170993 | Nucala 100 MG per 1 ML Auto-Injector | SY |
| 13 | 2173820 | mepolizumab 100 MG in 1 mL Prefilled Syringe | PSN |
| 14 | 2173820 | 1 ML mepolizumab 100 MG/ML Prefilled Syringe | SCD |
| 15 | 2173820 | mepolizumab 100 MG per 1 ML Prefilled Syringe | SY |
| 16 | 2173822 | Nucala 100 MG in 1 mL Prefilled Syringe | PSN |
| 17 | 2173822 | 1 ML mepolizumab 100 MG/ML Prefilled Syringe [Nucala] | SBD |
| 18 | 2173822 | 1 ML Nucala 100 MG/ML Prefilled Syringe | SY |
| 19 | 2173822 | Nucala 100 MG per 1 ML Prefilled Syringe | SY |
| 20 | 2596444 | mepolizumab 40 MG in 0.4 ML Prefilled Syringe | PSN |
| 21 | 2596444 | 0.4 ML mepolizumab 100 MG/ML Prefilled Syringe | SCD |
| 22 | 2596444 | mepolizumab 40 MG per 0.4 ML Prefilled Syringe | SY |
| 23 | 2596445 | Nucala 40 MG in 0.4 ML Prefilled Syringe | PSN |
| 24 | 2596445 | 0.4 ML mepolizumab 100 MG/ML Prefilled Syringe [Nucala] | SBD |
| 25 | 2596445 | 0.4 ML Nucala 100 MG/ML Prefilled Syringe | SY |
| 26 | 2596445 | Nucala 40 MG per 0.4 ML Prefilled Syringe | SY |
Get Label RSS Feed for this Drug
NUCALA- mepolizumab injection, powder, for solution
NUCALA- mepolizumab injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=fefb887c-e4ac-431e-8893-e9d1a5a63fea
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
NUCALA- mepolizumab injection, powder, for solution
NUCALA- mepolizumab injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0173-0881-01 |
| 2 | 0173-0881-61 |
| 3 | 0173-0892-01 |
| 4 | 0173-0892-42 |
| 5 | 0173-0892-61 |
| 6 | 0173-0892-63 |
| 7 | 0173-0904-42 |