Label: NOCDURNA- desmopressin acetate tablet
- NDC Code(s): 54436-325-10, 54436-325-30, 54436-350-10, 54436-350-30
- Packager: Antares Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NOCDURNA ® safely and effectively. See full prescribing information for NOCDURNA. NOCDURNA (desmopressin acetate) sublingual ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HYPONATREMIA
NOCDURNA can cause hyponatremia. Severe hyponatremia can be life-threatening, leading to seizures, coma, respiratory arrest, or death [see Warnings and Precautions (5.1)].
NOCDURNA is contraindicated in patients at increased risk of severe hyponatremia, such as patients with excessive fluid intake, illnesses that can cause fluid or electrolyte imbalances, and in those using loop diuretics or systemic or inhaled glucocorticoids [see Contraindications (4) and Warnings and Precautions (5.1)].
Ensure the serum sodium concentration is normal before starting or resuming NOCDURNA. Measure serum sodium within 7 days and approximately 1 month after initiating therapy, and periodically during treatment. More frequently monitor serum sodium in patients 65 years of age and older and in patients at increased risk of hyponatremia. [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
If hyponatremia occurs, NOCDURNA may need to be temporarily or permanently discontinued [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGENOCDURNA is indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least 2 times per night to void. In the NOCDURNA clinical trials nocturnal polyuria was ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Recommendations - Before starting or resuming NOCDURNA assess the sodium concentration and only start or resume NOCDURNA in patients with a normal serum sodium concentration [see ...
-
3 DOSAGE FORMS AND STRENGTHSSublingual tablets: 27.7 mcg of desmopressin acetate (equivalent to 25 mcg of desmopressin): White, round, with 25 on one side. 55.3 mcg of desmopressin acetate (equivalent to 50 mcg of ...
-
4 CONTRAINDICATIONSNOCDURNA is contraindicated in patients with the following conditions due to an increased risk of hyponatremia: Hyponatremia or a history of hyponatremia [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hyponatremia - NOCDURNA can cause hyponatremia [see Boxed Warning and Adverse Reactions (6.1)]. Severe hyponatremia can be life-threatening if it is not promptly diagnosed and treated ...
-
6 ADVERSE REACTIONSThe following adverse reaction is described elsewhere in the labeling: Hyponatremia [see Boxed Warning and Warnings and Precautions (5.1)] 6.1 Clinical Trial Experience - Because clinical ...
-
7 DRUG INTERACTIONS7.1 Drugs That May Increase the Risk of Hyponatremia - Concomitant use of NOCDURNA and loop diuretics or systemic or inhaled glucocorticoids is contraindicated because of the risk of severe ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - NOCDURNA is not recommended for the treatment of nocturia in pregnant women. Nocturia is usually related to normal, physiologic changes during pregnancy that do ...
-
10 OVERDOSAGEOverdosage of desmopressin leads to an increased risk of prolonged fluid retention and hyponatremia. Signs of overdosage may include nausea, headache, drowsiness, confusion, and rapid weight gain ...
-
11 DESCRIPTIONNOCDURNA is a sublingual tablet containing desmopressin acetate, a synthetic analog of the endogenous pituitary hormone, 8-arginine vasopressin (ADH), an antidiuretic hormone. It is chemically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The antidiuretic effects of desmopressin are mediated by stimulation of vasopressin 2 (V2) receptors, thereby increasing water re-absorption in the kidneys, and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies with desmopressin have not been performed to evaluate carcinogenic potential. Desmopressin was not mutagenic in bacterial ...
-
14 CLINICAL STUDIESThe efficacy of NOCDURNA in the treatment of adults with nocturia due to nocturnal polyuria was established in two 3-month randomized, double-blind, placebo-controlled, multicenter trials in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNOCDURNA (desmopressin acetate) sublingual tablets are available as: 27.7 mcg of desmopressin acetate (equivalent to 25 mcg of desmopressin): White, round, sublingual tablet with "25" on one ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Fluid Restriction, Hyponatremia, Sodium Monitoring and Acute Illnesses - Instruct patients to place one tablet ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Antares Pharma, Inc. Ewing, NJ 08628 - Origin Sweden - 2009055896 - LB-0153V0
-
MEDICATION GUIDEMEDICATION GUIDE - NOCDURNA® (knock-DUHR-nah) (desmopressin acetate) sublingual tablets - What is the most important information I should know about NOCDURNA? NOCDURNA may cause serious side ...
-
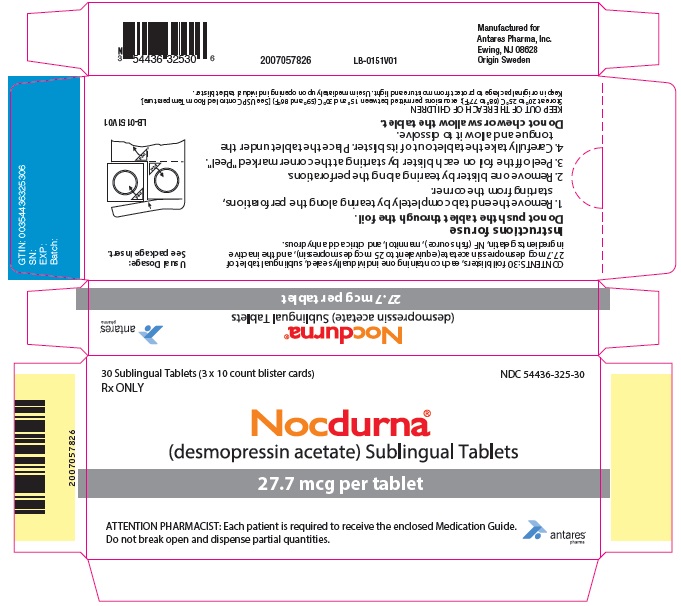
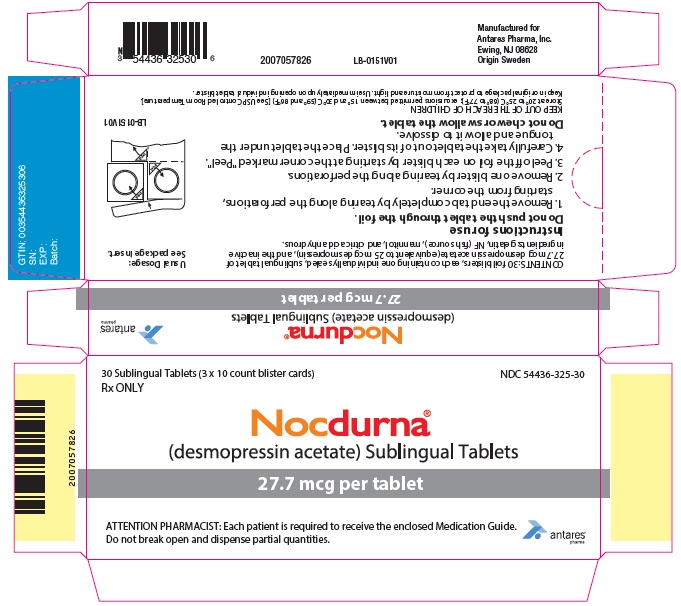
PRINCIPAL DISPLAY PANEL - 27.7 mcg Tablet Blister Pack Carton30 Sublingual Tablets (3 x 10 count blister cards) Rx ONLY - NDC 54436-325-30 - Nocdurna ® (desmopressin acetate) Sublingual Tablets - 27.7 mcg per tablet - ATTENTION PHARMACIST: Each ...
-
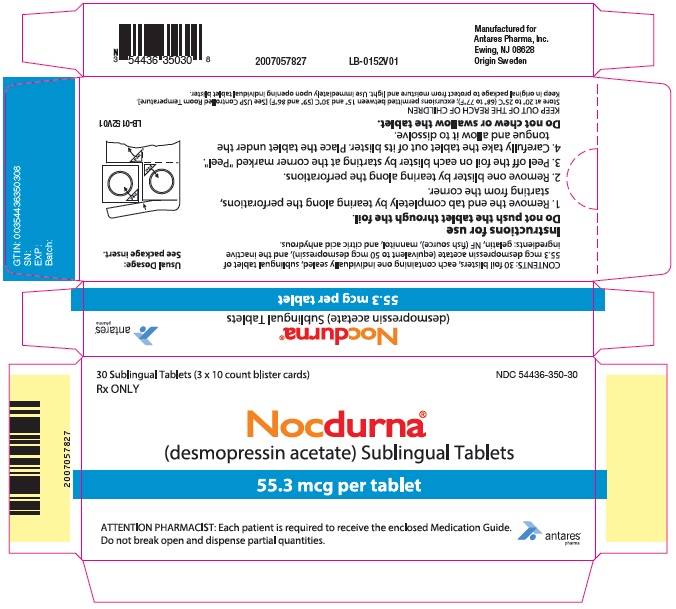
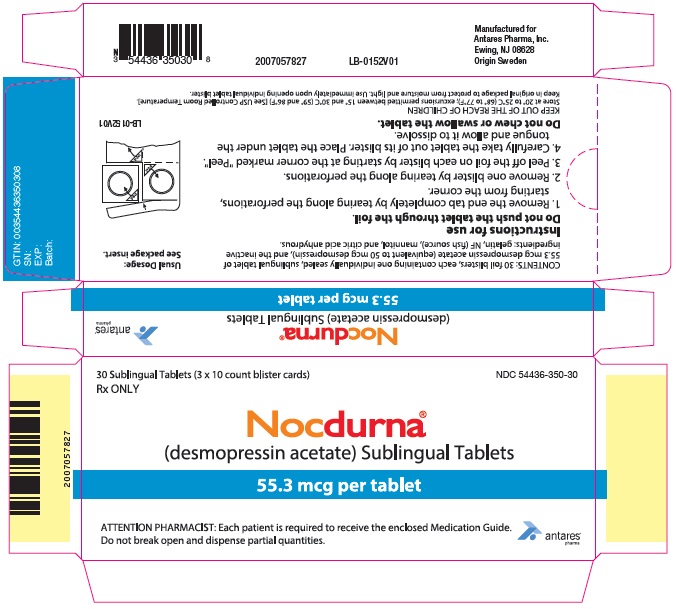
PRINCIPAL DISPLAY PANEL - 55.3 mcg Tablet Blister Pack Carton30 Sublingual Tablets (3 x 10 count blister cards) Rx ONLY - NDC 54436-350-30 - Nocdurna® (desmopressin acetate) Sublingual Tablets - 55.3 mcg per tablet - ATTENTION PHARMACIST: Each patient is ...
-
INGREDIENTS AND APPEARANCEProduct Information