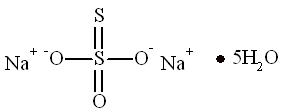Label: NITHIODOTE- sodium nitrite and sodium thiosulfate kit
- NDC Code(s): 60267-311-10, 60267-705-50, 60267-812-00
- Packager: Hope Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use the NITHIODOTE safely and effectively. See full prescribing information for NITHIODOTE. NITHIODOTE (sodium nitrite injection and ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: LIFE THREATENING HYPOTENSION AND METHEMOGLOBIN FORMATION
Sodium nitrite can cause serious adverse reactions and death in humans, even at doses less than twice the recommended therapeutic dose. Sodium nitrite causes hypotension and methemoglobin formation, which diminishes oxygen carrying capacity. Hypotension and methemoglobin formation can occur concurrently or separately. Because of these risks, sodium nitrite should be used to treat acute life-threatening cyanide poisoning and be used with caution in patients where the diagnosis of cyanide poisoning is uncertain.
Patients should be closely monitored to ensure adequate perfusion and oxygenation during treatment with sodium nitrite.
Alternative therapeutic approaches should be considered in patients known to have diminished oxygen or cardiovascular reserve (e.g. smoke inhalation victims, pre-existing anemia, cardiac or respiratory compromise), and those at higher risk of developing methemoglobinemia (e.g., congenital methemoglobin reductase deficiency) as they are at greater risk for potentially life-threatening adverse events related to the use of sodium nitrite. [See Warnings and Precautions (5.1 and 5.2)]
Close -
1 INDICATIONS AND USAGENITHIODOTE is indicated for the treatment of acute cyanide poisoning that is judged to be serious or life-threatening. When the diagnosis of cyanide poisoning is uncertain, carefully weigh the ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Instructions - If clinical suspicion of cyanide poisoning is high, administer NITHIODOTE without delay. Comprehensive treatment of acute cyanide ...
-
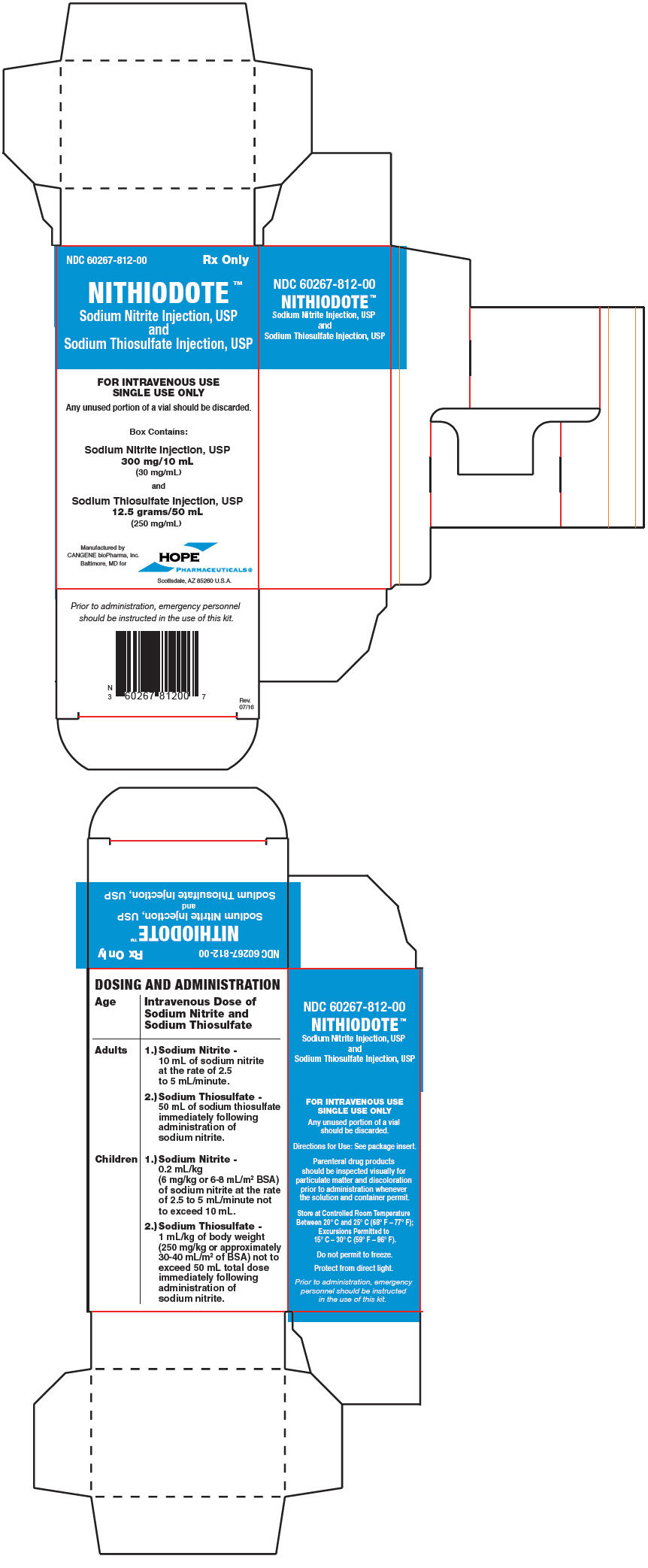
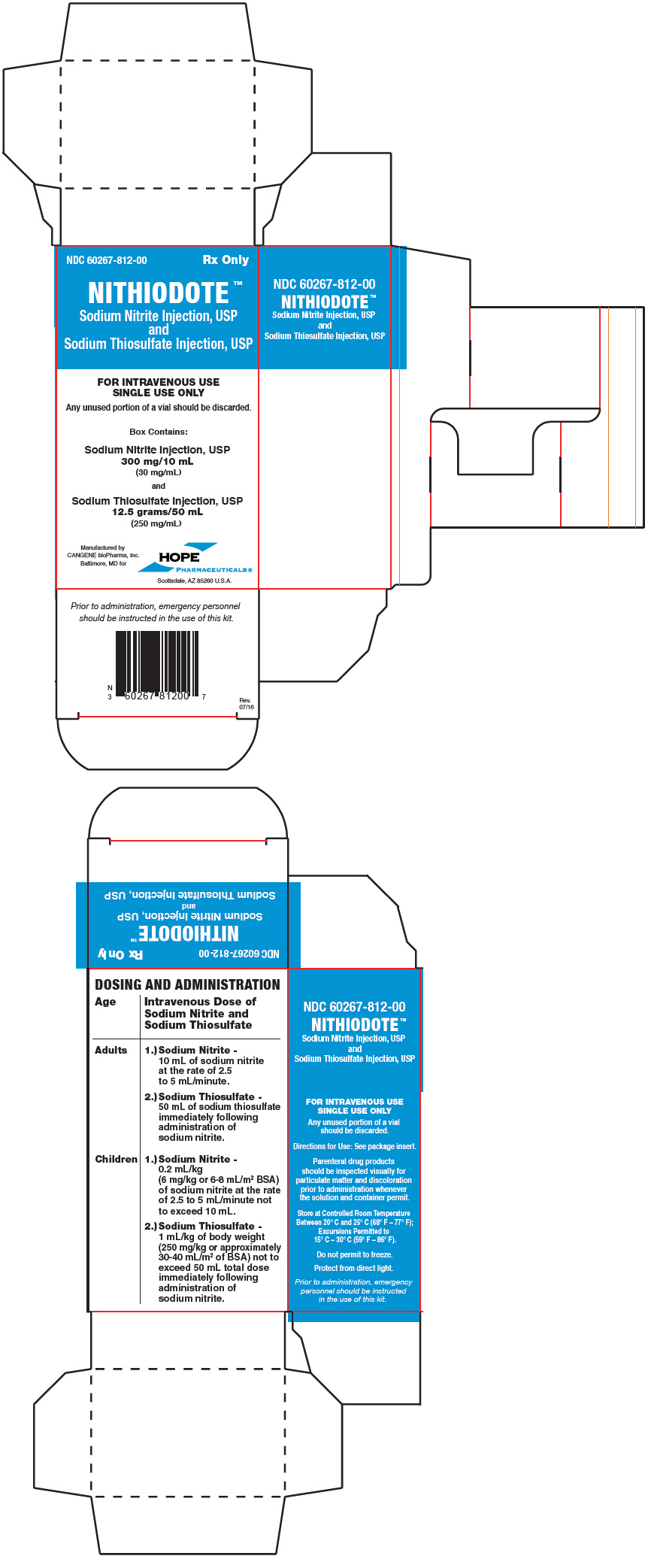
3 DOSAGE FORMS AND STRENGTHSNITHIODOTE Injection consists of: One vial of sodium nitrite injection, USP 300 mg/10 mL (30 mg/mL) and - One vial of sodium thiosulfate injection USP 12.5 grams/50 mL (250 mg/mL) Administration ...
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Sodium nitrite has been associated with severe hypotension, methemoglobinemia, and death at doses less than twice recommended therapeutic doses. Hypotension may occur ...
-
6 ADVERSE REACTIONSThere have been no controlled clinical trials conducted to systematically assess the adverse events profile of sodium nitrite or sodium thiosulfate. The medical literature has reported the ...
-
7 DRUG INTERACTIONSFormal drug interaction studies have not been conducted with NITHIODOTE.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Life-sustaining therapy should not be withheld. Cyanide poisoning is a medical emergency in pregnancy, which can be fatal for the pregnant woman and fetus if ...
-
10 OVERDOSAGESodium Nitrite - Large doses of sodium nitrite result in severe hypotension and toxic levels of methemoglobin which may lead to cardiovascular collapse. Sodium nitrite administration has been ...
-
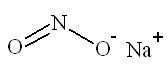
11 DESCRIPTIONSodium nitrite, one of the active ingredients in NITHIODOTE has the chemical name nitrous acid sodium salt. The chemical formula is NaNO2 and the molecular weight is 69.0. Sodium thiosulfate, the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Cyanide is an extremely toxic poison. In the absence of rapid and adequate treatment, exposure to a high dose of cyanide can result in death within minutes due to the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Sodium Nitrite - The potential benefit of an acute exposure to sodium nitrite as part of a cyanide antidote ...
-
14 CLINICAL STUDIESHuman Data - The human data supporting the use of sodium thiosulfate for cyanide poisoning consists primarily of published case reports. There are no randomized controlled clinical trials ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach NITHIODOTE carton (NDC 60267-812-00) consists of the following: One 10 mL glass vial of sodium nitrite injection 30 mg/mL (containing 300 mg of sodium nitrite); One 50 mL glass vial of ...
-
17 PATIENT COUNSELING INFORMATIONNITHIODOTE is indicated for cyanide poisoning and in this setting, patients will likely be unresponsive or may have difficulty in comprehending counseling information. Hypotension and ...
-
SPL UNCLASSIFIED SECTIONHope Pharmaceuticals, Scottsdale, Arizona 85260 - US PATENTS 8496973, 8568793, 9345724, and 9585912
-
PRINCIPAL DISPLAY PANEL - Kit CartonNDC 60267-812-00 - Rx Only - NITHIODOTE™ Sodium Nitrite Injection, USP - and - Sodium Thiosulfate Injection, USP - FOR INTRAVENOUS USE - SINGLE USE ONLY - Any unused portion of a vial should be discarded. Box ...
-
INGREDIENTS AND APPEARANCEProduct Information