Label: NIPRIDE RTU- sodium nitroprusside injection, solution
- NDC Code(s): 51754-1006-1, 51754-1018-1, 51754-1029-1
- Packager: EXELA PHARMA SCIENCES, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 20, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NIPRIDE® RTU safely and effectively. See full prescribing information for NIPRIDE® RTU. NIPRIDE® RTU, for intravenous use - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: (A) EXCESSIVE HYPOTENSION; (B) CYANIDE TOXICITY
(A) EXCESSIVE HYPOTENSION:
Sodium Nitroprusside can cause precipitous decreases in blood pressure which can lead to irreversible ischemic injuries or death. Use only with continuous blood pressure monitoring [see Dosage and Administration (2.2) and Warnings and Precautions (5.1).
(B) CYANIDE TOXICITY:
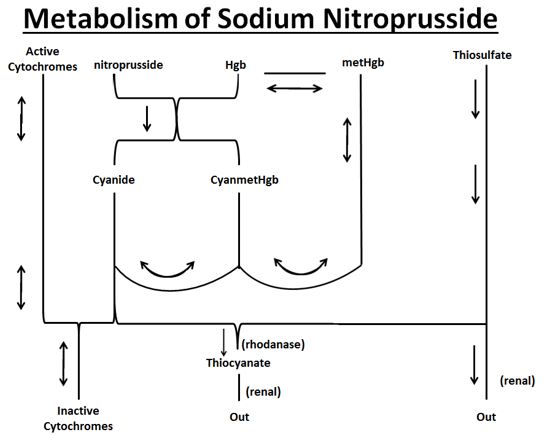
Sodium nitroprusside metabolism produces dose-related cyanide, which can be lethal. A patient’s ability to buffer cyanide will be exceeded in less than one hour at the maximum dose rate (10 mcg/kg/min); limit infusions at the maximum rate to as short a duration as possible [see Warnings and Precautions (5.2)].
Close -
1 INDICATIONS AND USAGE1.1 Immediate Reduction of Blood Pressure - Sodium nitroprusside is indicated for the immediate reduction of blood pressure of adult and pediatric patients in hypertensive crises. 1.2 ...
-
2 DOSAGE AND ADMINISTRATION2.1 Inspection - Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. Sodium nitroprusside should be a clear ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 50 mg/100 mL of 0.9% sodium chloride (0.5 mg/mL), 20 mg/100 mL of 0.9% sodium chloride (0.2 mg/mL), and 10 mg/50 mL of 0.9% sodium chloride (0.2 mg/mL). NIPRIDE® RTU is supplied as a ...
-
4 CONTRAINDICATIONS• Diseases with compensatory hypertension (e.g., coarctation of the aorta, arteriovenous shunting). • Inadequate cerebral circulation or in moribund patients (A.S.A. Class 5E) coming to emergency ...
-
5 WARNINGS AND PRECAUTIONS5.1 Excessive Hypotension - Sodium nitroprusside, can cause excessive hypotension leading to hypoperfusion of vital organs. Hypotension should resolve within 1-10 minutes after discontinuation of ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described, or described in greater detail, in other sections: • Hypotension [see Warnings and Precautions (5.1)] • Cyanide Toxicity [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on animal data and mechanism of action, sodium nitroprusside may lead to cyanide exposure and potential adverse effects in the fetus [see Clinical ...
-
10 OVERDOSAGEOverdosage of nitroprusside can be manifested as excessive hypotension or cyanide toxicity [see Warnings and Precaution (5.1, 5.2)] or as thiocyanate toxicity [see Warnings and Precautions (5.3)] ...
-
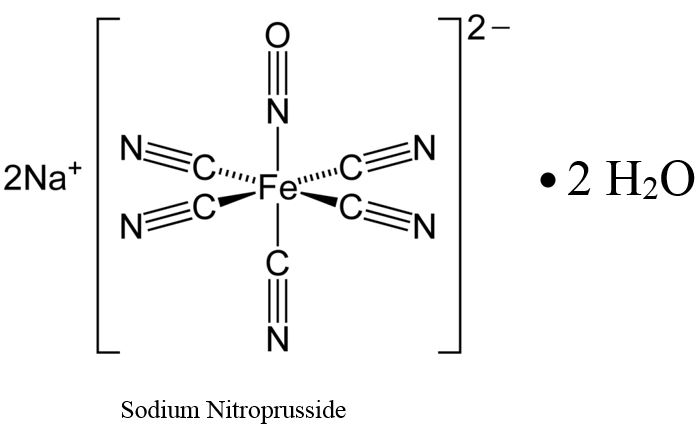
11 DESCRIPTIONSodium nitroprusside is disodium pentacyanonitrosylferrate(2-) dihydrate, a hypotensive agent whose structural formula is - Sodium Nitroprusside has molecular formula Na2[Fe(CN)5NO] • 2H2O and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sodium nitroprusside interacts with oxyhemoglobin to produce methemoglobin, cyanide, and nitric oxide (NO). NO then reacts with guanylate cyclase in vascular smooth ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies assessing sodium nitroprusside’s carcinogenicity and mutagenicity have not been conducted. Similarly, sodium ...
-
14 CLINICAL STUDIESBaseline-controlled clinical trials have uniformly shown that sodium nitroprusside has a prompt hypotensive effect, at least initially, in all populations. With increasing rates of infusion ...
-
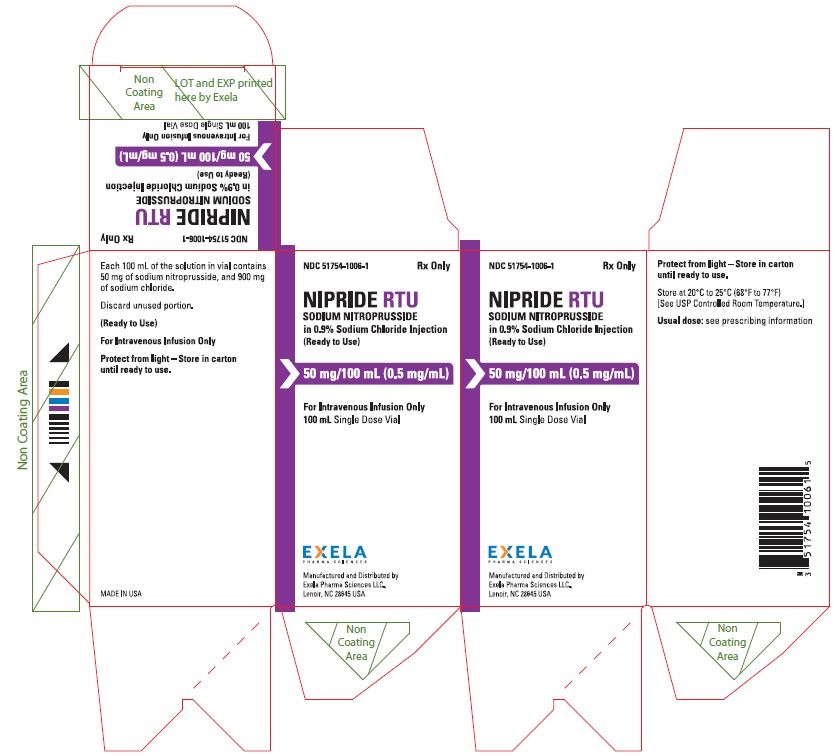
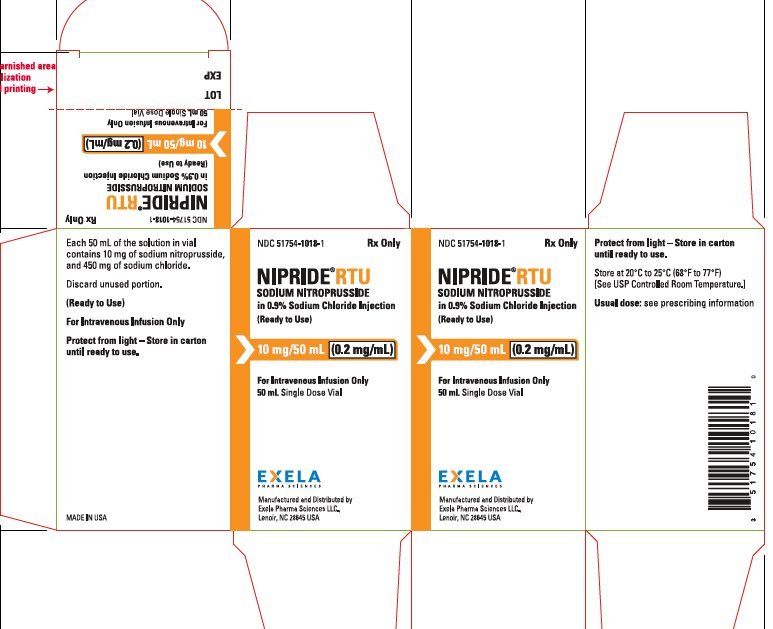
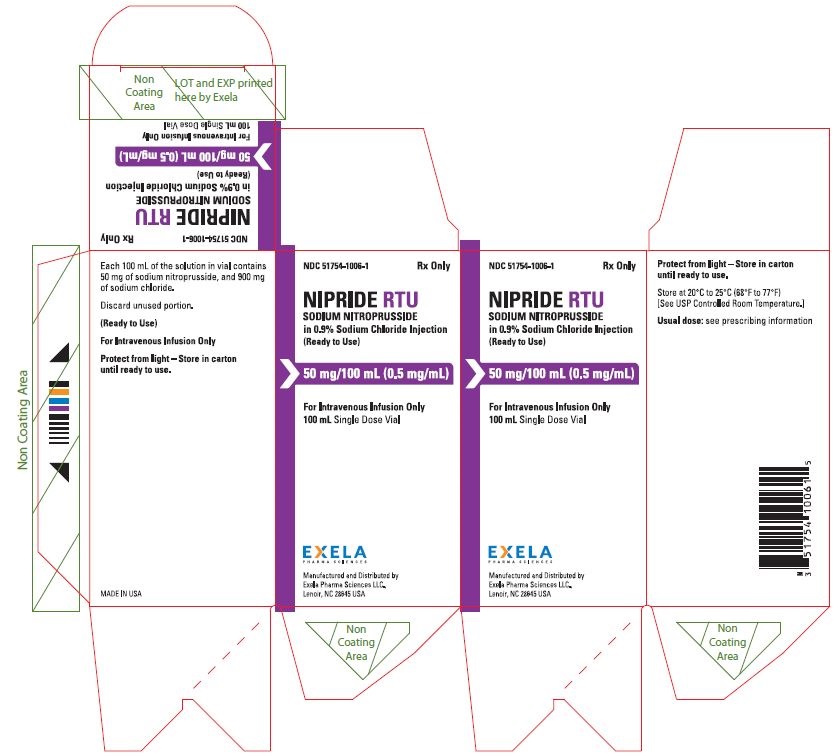
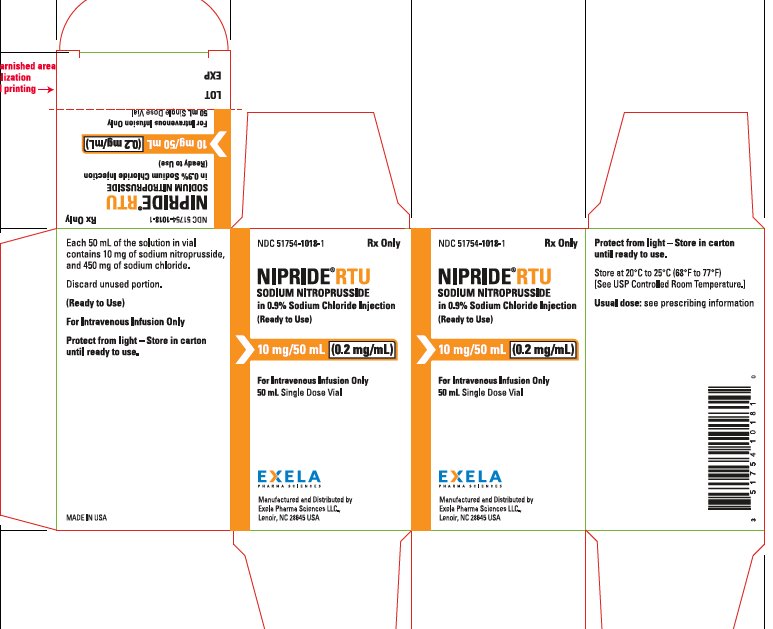
16 HOW SUPPLIED/STORAGE AND HANDLINGNIPRIDE®RTU is supplied in amber-colored, single-dose, 50 mg/100 mL (0.5 mg/mL) Fliptop Vials (NDC 51754-1006-1), 20 mg/100 mL (0.2 mg/mL) Fliptop Vials (NDC 51754-1029-1) and 10 mg/50 mL (0.2 ...
-
17 PATIENT COUNSELING INFORMATIONPregnancy - Inform female patients of reproductive potential that Sodium Nitroprusside Injection may cause fetal harm and to inform their prescriber of a known or suspected pregnancy [see Use in ...
-
SPL UNCLASSIFIED SECTIONManufactured and Distributed by: Exela Pharma Sciences, LLC - Lenoir, NC 28645
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- 0.5 mg/mL in 100 mL Vial Label Rx Only NDC 51754-1006-1 - NIPRIDE® RTU - SODIUM NITROPRUSSIDE - in 0.9% Sodium Chloride Injection - (Ready to Use) 50 mg/100mL (0.5 mg/mL) For Intravenous Infusion Only - 100 mL Single ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-0.5 mg/mL in 100 mL Carton Rx Only NDC 51754-1018-1 - NIPRIDE RTU - SODIUM NITROPRUSSIDE - in 0.9% Sodium Chloride Injection - (Ready to Use) 10 mg/50mL (0.2 mg/mL) For Intravenous Infusion Only - 50 mL ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-0.2 mg/mL in 50 mL Vial Label NDC 51754-1018-1 Rx Only - NIPRIDE® RTU - SODIUM NITROPRUSSIDE - in 0.9% Sodium Chloride Injection - (Ready to Use) 10 mg/50mL (0.2 mg/mL) For Intravenous Infusion Only - 50 mL ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL0.2 mg/mL in 50 mL Carton NDC 51754-1018-1 Rx Only - NIPRIDE® RTU - SODIUM NITROPRUSSIDE - in 0.9% Sodium Chloride Injection - (Ready to Use) 10 mg/50mL (0.2 mg/mL) For Intravenous Infusion Only - 50 mL ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-0.2 mg/mL in 20 mL Vial Label NDC 51754-1029-1 Rx Only - NIPRIDE® RTU - SODIUM NITROPRUSSIDE - in 0.9% Sodium Chloride Injection - (Ready to Use) 20 mg/100mL (0.2 mg/mL) For Intravenous Infusion Only - 100 mL ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-0.2 mg/mL in 20 mL Carton NDC 51754-1029-1 Rx Only - NIPRIDE® RTU - SODIUM NITROPRUSSIDE - in 0.9% Sodium Chloride Injection - (Ready to Use) 20 mg/100mL (0.2 mg/mL) For Intravenous Infusion Only - 100 mL ...
-
INGREDIENTS AND APPEARANCEProduct Information