Label: NIPENT- pentostatin injection, powder, lyophilized, for solution
- NDC Code(s): 0409-0801-01
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONNIPENT™ (pentostatin for injection)
-
SPL UNCLASSIFIED SECTIONRx only
-
BOXED WARNING
(What is this?)
WARNING
NIPENT should be administered under the supervision of a physician qualified and experienced in the use of cancer chemotherapeutic agents. The use of higher doses than those specified (see DOSAGE AND ADMINISTRATION) is not recommended. Dose-limiting severe renal, liver, pulmonary, and CNS toxicities occurred in Phase 1 studies that used NIPENT at higher doses (20-50 mg/m2 in divided doses over 5 days) than recommended.
In a clinical investigation in patients with refractory chronic lymphocytic leukemia using NIPENT at the recommended dose in combination with fludarabine phosphate, 4 of 6 patients entered in the study had severe or fatal pulmonary toxicity. The use of NIPENT in combination with fludarabine phosphate is not recommended.
Close -
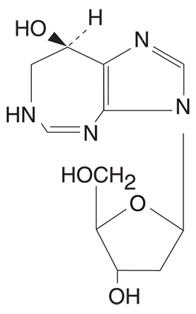
DESCRIPTIONNIPENT™ (pentostatin for injection) is supplied as a sterile, apyrogenic, lyophilized powder in single-dose vials for intravenous administration. Each vial contains 10 mg of pentostatin and 50 mg ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Pentostatin is a potent transition state inhibitor of the enzyme adenosine deaminase (ADA). The greatest activity of ADA is found in cells of the lymphoid system with T-cells ...
-
CLINICAL STUDIESThe following table provides efficacy results for 4 groups (columns) of patients with hairy cell leukemia: patients who initially received NIPENT, patients who initially received alpha-interferon ...
-
INDICATIONS AND USAGENIPENT is indicated as single-agent treatment for both untreated and alpha-interferon-refractory hairy cell leukemia patients with active disease as defined by clinically significant anemia ...
-
CONTRAINDICATIONSNIPENT is contraindicated: In patients who have demonstrated hypersensitivity to NIPENT.
-
WARNINGSSee Boxed Warning. Patients with hairy cell leukemia may experience myelosuppression primarily during the first few courses of treatment. Patients with infections prior to NIPENT treatment have in ...
-
PRECAUTIONSGeneral - Therapy with NIPENT requires regular patient observation and monitoring of hematologic parameters and blood chemistry values. If severe adverse reactions occur, the drug should be ...
-
ADVERSE REACTIONSMost patients treated for hairy cell leukemia in the five NCI-sponsored Phase 2 studies and the Phase 3 SWOG study experienced an adverse event. The following table lists the most frequently ...
-
OVERDOSAGENo specific antidote for NIPENT overdose is known. NIPENT administered at higher doses (20- 50 mg/m2 in divided doses over 5 days) than recommended was associated with deaths due to severe renal ...
-
DOSAGE AND ADMINISTRATIONIt is recommended that patients receive hydration with 500 to 1,000 mL of 5% Dextrose in 0.5 Normal Saline or equivalent before NIPENT administration. An additional 500 mL of 5% Dextrose or ...
-
HOW SUPPLIEDNIPENT (pentostatin for injection) is supplied as a sterile lyophilized white to off-white powder in single-dose vials containing 10 mg of pentostatin. The vials are packed in individual cartons ...
-
REFERENCES1. Malspeis L, et al. Clinical pharmacokinetics of 2'-Deoxycoformycin. Cancer Treatment Symposia 2:7-15, 1984 - 2. Recommendations for the safe handling of parenteral antineoplastic drugs. NIH ...
-
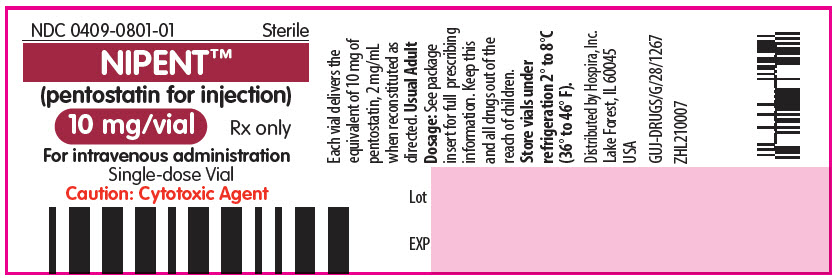
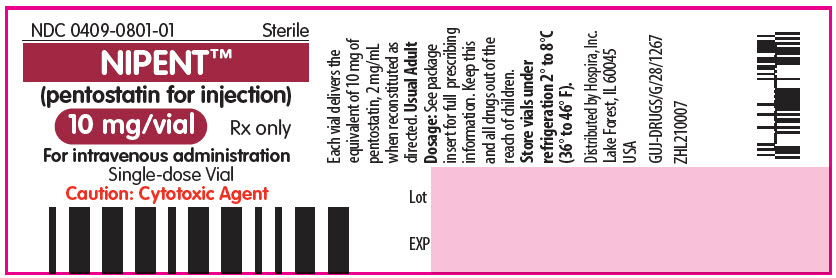
PRINCIPAL DISPLAY PANEL - 10 mg Vial LabelNDC 0409-0801-01 - Sterile - NIPENT™ (pentostatin for injection) 10 mg/vial - Rx only - For intravenous administration - Single-dose Vial - Caution: Cytotoxic Agent
-
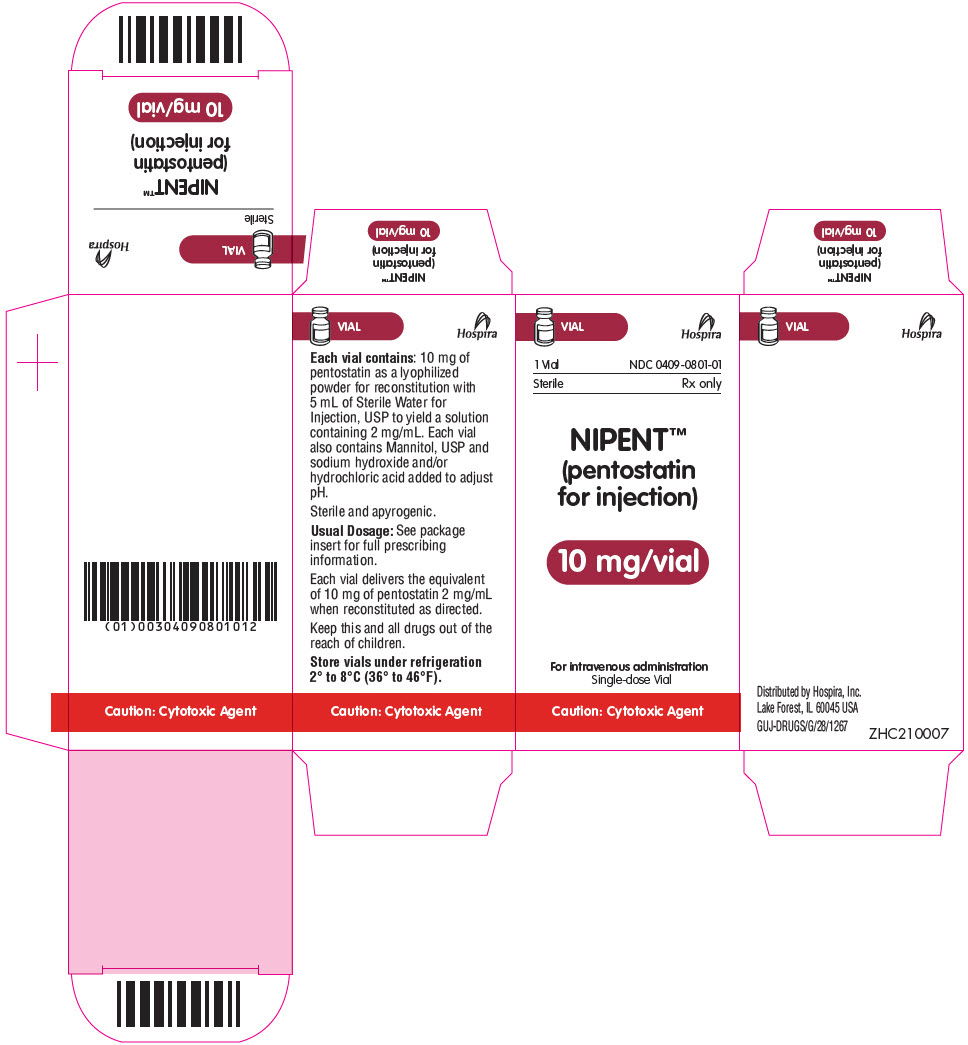
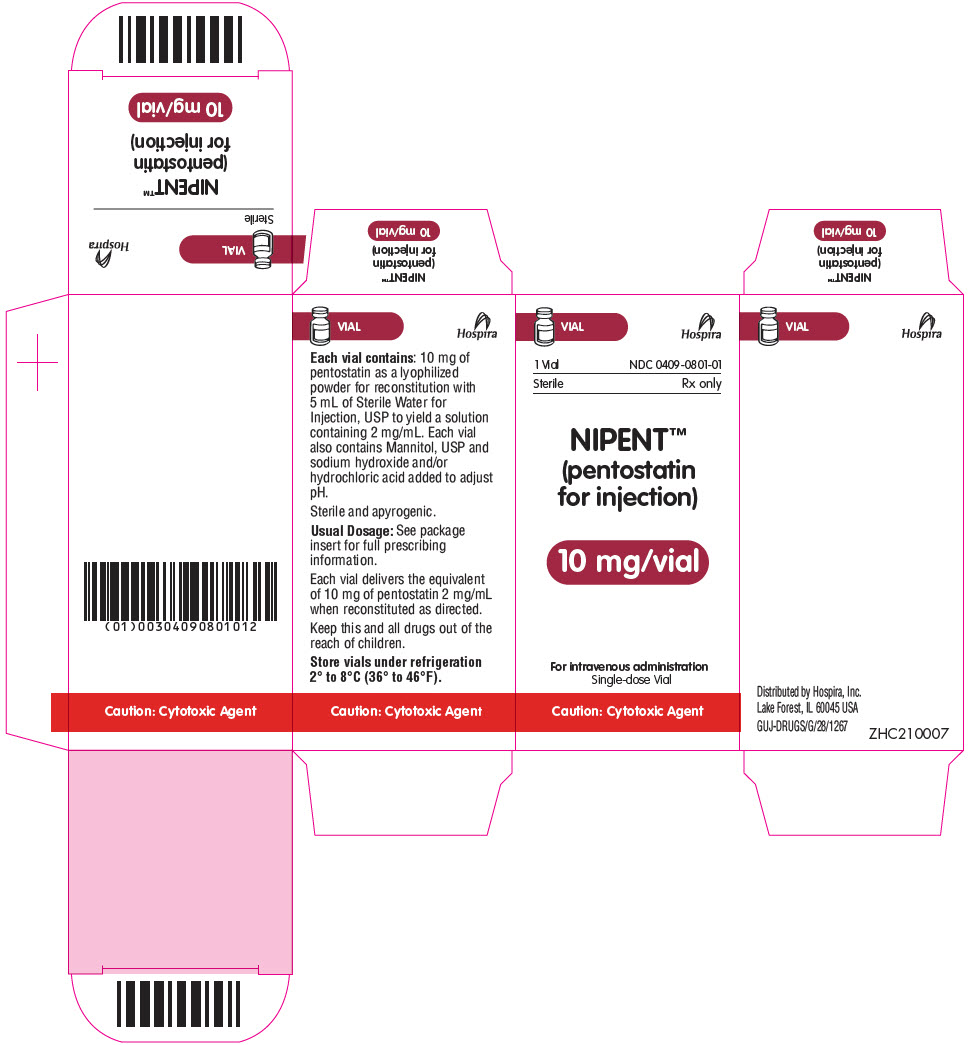
PRINCIPAL DISPLAY PANEL - 10 mg Vial CartonVIAL - Hospira - 1 Vial - NDC 0409-0801-01 - Sterile - Rx only - NIPENT™ (pentostatin - for injection) 10 mg/vial - For intravenous administration - Single-dose Vial - Caution: Cytotoxic Agent
-
INGREDIENTS AND APPEARANCEProduct Information