Label: NILANDRON- nilutamide tablet
- NDC Code(s): 59212-111-14
- Packager: Advanz Pharma (US) Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
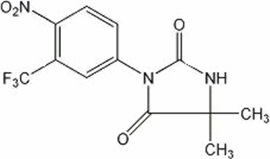
DESCRIPTIONNILANDRON® tablets contain nilutamide, a nonsteroidal, orally active antiandrogen having the chemical name 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]-2,4-imidazolidinedione with the ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Prostate cancer is known to be androgen sensitive and responds to androgen ablation. In animal studies, nilutamide has demonstrated antiandrogenic activity without other ...
-
INDICATIONS AND USAGEMetastatic Prostate Cancer - NILANDRON tablets are indicated for use in combination with surgical castration for the treatment of metastatic prostate cancer (Stage D2). For maximum benefit ...
-
CONTRAINDICATIONSNILANDRON tablets are contraindicated: in patients with severe hepatic impairment (baseline hepatic enzymes should be evaluated prior to treatment ...
-
WARNINGSInterstitial Pneumonitis - Interstitial pneumonitis has been reported in 2% of patients in controlled clinical trials in patients exposed to nilutamide. A small study in Japanese subjects showed ...
-
PRECAUTIONSGeneral - Antiandrogen Withdrawal Syndrome - Patients whose disease progresses while being treated with an antiandrogen may experience clinical improvement with discontinuation of the ...
-
ADVERSE REACTIONSClinical Trial Experience - The following adverse experiences were reported during a multicenter clinical trial comparing NILANDRON + surgical castration versus placebo + surgical castration ...
-
OVERDOSAGEOne case of massive overdosage has been published. A 79-year-old man attempted suicide by ingesting 13 g of nilutamide (i.e., 43 times the maximum recommended dose). Despite immediate gastric ...
-
DOSAGE AND ADMINISTRATIONThe recommended dosage is 300 mg once a day for 30 days, followed thereafter by 150 mg once a day. NILANDRON tablets can be taken with or without food.
-
HOW SUPPLIEDNILANDRON 150 mg tablets are supplied in boxes of 30 tablets. Each box contains 3 child-resistant, PVC, aluminum foil-backed blisters of 10 tablets (NDC 59212-111-14). Each white, biconvex ...
-
PRINCIPAL DISPLAY PANELNDC 59212-111-14 - Nilandron® 150 mg - nilutamide - Tablets - 150 mg - 30 Tablets - ADVANZ PHARMA
-
INGREDIENTS AND APPEARANCEProduct Information