Label: NEXLETOL- bempedoic acid tablet, film coated
- NDC Code(s): 72426-118-03, 72426-118-09, 72426-118-99
- Packager: Esperion Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 24, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NEXLETOL® safely and effectively. See full prescribing information for NEXLETOL. NEXLETOL (bempedoic acid) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENEXLETOL is indicated: To reduce the risk of myocardial infarction and coronary revascularization in adults who are unable to take recommended statin therapy (including those not taking a ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of NEXLETOL is 180 mg administered orally once daily. NEXLETOL can be taken with or without food. After initiation of NEXLETOL, analyze lipid ...
-
3 DOSAGE FORMS AND STRENGTHSNEXLETOL is available as: Tablets: 180 mg, white to off-white, oval shaped, debossed with "180" on one side and "ESP" on the other side.
-
4 CONTRAINDICATIONSNEXLETOL is contraindicated in patients with a prior serious hypersensitivity reaction to bempedoic acid or any of the excipients in NEXLETOL. Serious hypersensitivity reactions, such as ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hyperuricemia - NEXLETOL inhibits renal tubular OAT2 and may increase blood uric acid levels [see Clinical Pharmacology (12.3)]. In the primary hyperlipidemia trials [see Clinical Studies ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hyperuricemia [see Warnings and Precautions (5.1)] Tendon Rupture [see Warnings and Precautions ...
-
7 DRUG INTERACTIONSTable 3 includes a list of drugs with clinically important drug interactions when administered concomitantly with NEXLETOL and instructions for preventing or managing them. Table 3. Clinically ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Discontinue NEXLETOL when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus. There are insufficient data on ...
-
10 OVERDOSAGEThere is no clinical experience with NEXLETOL overdose. In the event of an overdosage, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional ...
-
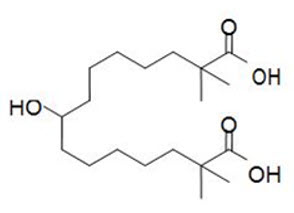
11 DESCRIPTIONNEXLETOL tablets, for oral use, contain bempedoic acid, an adenosine triphosphate-citrate lyase (ACL) inhibitor. The chemical name for bempedoic acid is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bempedoic acid is an adenosine triphosphate-citrate lyase (ACL) inhibitor that lowers LDL-C by inhibition of cholesterol synthesis in the liver. ACL is an enzyme ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Bempedoic acid was negative for mutagenicity in an in vitro Ames assay and negative for clastogenicity in the vitro human lymphocyte ...
-
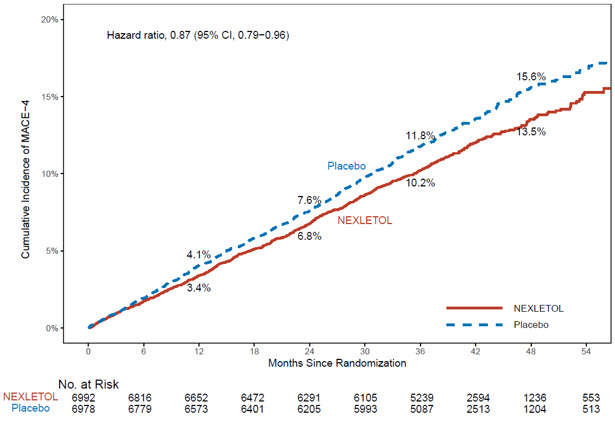
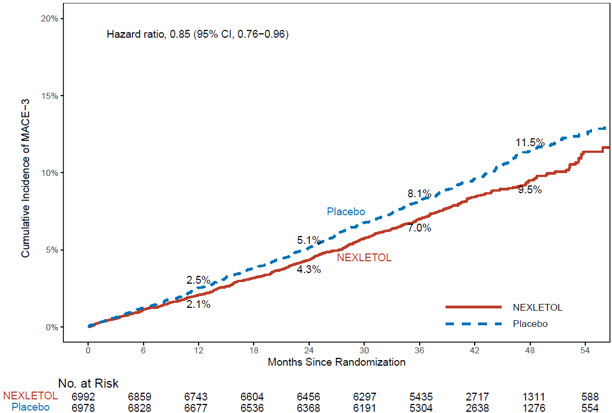
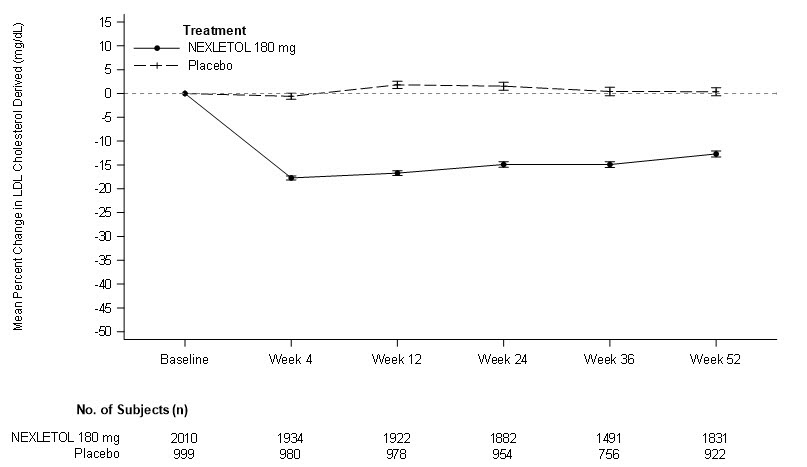
14 CLINICAL STUDIES14.1 Cardiovascular Outcomes Trial in Adults With CVD or at High Risk for CVD - Trial 1 (NCT02993406) was a randomized, double-blind, placebo-controlled, event-driven trial in 13,970 adult ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - NEXLETOL tablets are supplied as follows: Tablet StrengthDescriptionPackage ConfigurationNDC No. 180 mgWhite to off white and oval, debossed with "180" on one side ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information). Risk of Hyperuricemia - Advise patients of the risk of elevated serum uric acid levels, including development of ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Esperion Therapeutics, Inc. 3891 Ranchero Drive, Suite 150 - Ann Arbor, MI 48108 - NEXLETOL® (bempedoic acid) tablets - © 2024 Esperion Therapeutics, Inc.
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - NEXLETOL® (NEX-le-tol) (bempedoic acid) tablets, for oral use - This Patient Information has been approved by the U.S. Food and Drug AdministrationRevised ...
-
PRINCIPAL DISPLAY PANEL - 180 mg Tablet Bottle LabelNDC 72426-118-03 - Rx only - NEXLETOL® (bempedoic acid) tablets - Contains - 30 Tablets - 180 mg
-
INGREDIENTS AND APPEARANCEProduct Information