Label: NEXESTA FE- norethindrone and ethinyl estradiol and ferrous fumarate kit
- NDC Code(s): 65862-926-58, 65862-926-87, 65862-926-88, 65862-926-97
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 4, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
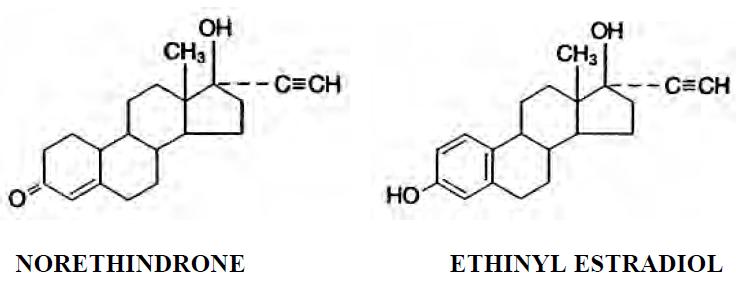
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NEXESTATM Fe safely and effectively. See Full Prescribing Information for NEXESTA Fe. NEXESTA Fe (norethindrone and ethinyl ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs are contraindicated in women who are over 35 years of age and smoke [see Contraindications (4)].
Close -
1 INDICATIONS AND USAGENexesta Fe is indicated for use by females of reproductive potential to prevent pregnancy.
-
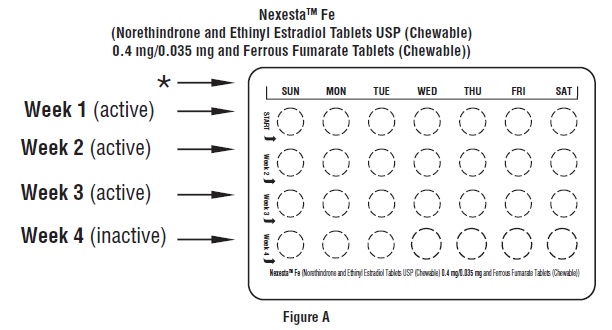
2 DOSAGE AND ADMINISTRATION2.1 How to Start Nexesta Fe - Nexesta Fe is dispensed in a blister pack [see How Supplied/Storage and Handling (16)]. Nexesta Fe may be started using either a Day 1 start or a Sunday start (see ...
-
3 DOSAGE FORMS AND STRENGTHSNexesta Fe (norethindrone and ethinyl estradiol tablets USP (chewable) and ferrous fumarate tablets (chewable)) is available in blister packs. Each blister pack contains 28 tablets in the ...
-
4 CONTRAINDICATIONSNexesta Fe is contraindicated in females who are known to have or develop the following conditions: • A high risk of arterial or venous thrombotic diseases. Examples include women who are known ...
-
5 WARNINGS AND PRECAUTIONS5.1 Thrombotic Disorders and Other Vascular Problems - • Stop Nexesta Fe if an arterial thrombotic event or venous thromboembolic (VTE) event occurs. • Stop Nexesta Fe if there is unexplained ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling: Serious cardiovascular events and stroke [see Boxed Warning and Warnings and Precautions ...
-
7 DRUG INTERACTIONSConsult the labeling of concurrently used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations. 7.1 Effects of Other Drugs ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - There is little or no increased risk of birth defects in women who inadvertently use COCs during early pregnancy. Epidemiologic studies and meta-analyses have not found an ...
-
10 OVERDOSAGEThere have been no reports of serious ill effects from overdosage of oral contraceptives, including ingestion by children. Overdosage may cause withdrawal bleeding in females and nausea.
-
11 DESCRIPTIONNexesta Fe is a combinational contraceptive containing the progestational compound norethindrone and the estrogenic compound ethinyl estradiol. The packaging includes 21 white to off-white tablets ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - COCs lower the risk of becoming pregnant primarily by suppressing ovulation. Other possible mechanisms may include cervical mucus changes that inhibit sperm penetration ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - [See Warnings and Precautions (5.11) and Use in Specific Populations (8.1).]
-
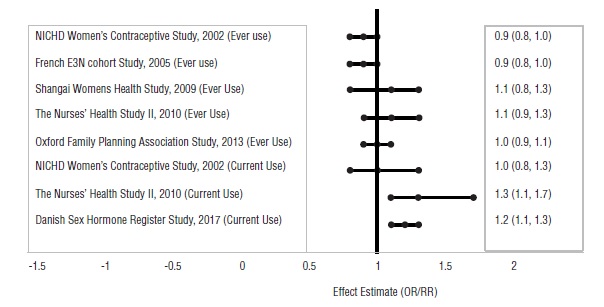
14 CLINICAL STUDIESThe data presented in Section 14 are from a clinical trial conducted with norethindrone 0.4 mg/ethinyl estradiol 0.035 mg tablets. Nexesta Fe is bioequivalent to these norethindrone ...
-
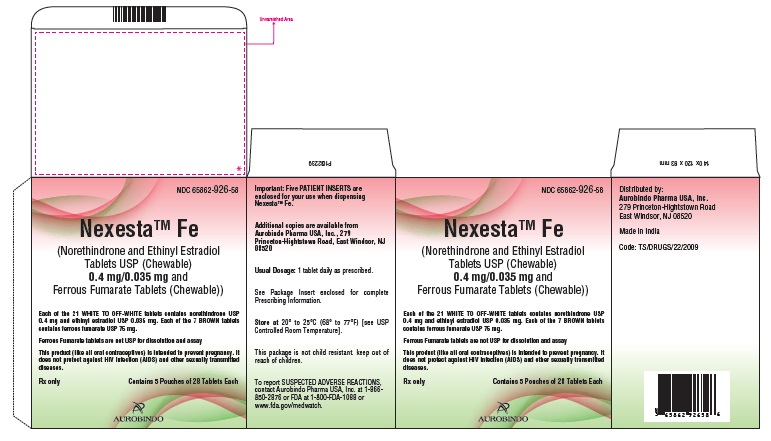
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Nexesta Fe (norethindrone and ethinyl estradiol tablets USP (chewable) 0.4 mg/0.035 mg and ferrous fumarate tablets (chewable)) is available in blister packs: The Blister ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-Approved Patient Labeling (Patient Information and Instructions for Use) Counsel patients about the following information: Cigarette smoking increases the risk of serious cardiovascular ...
-
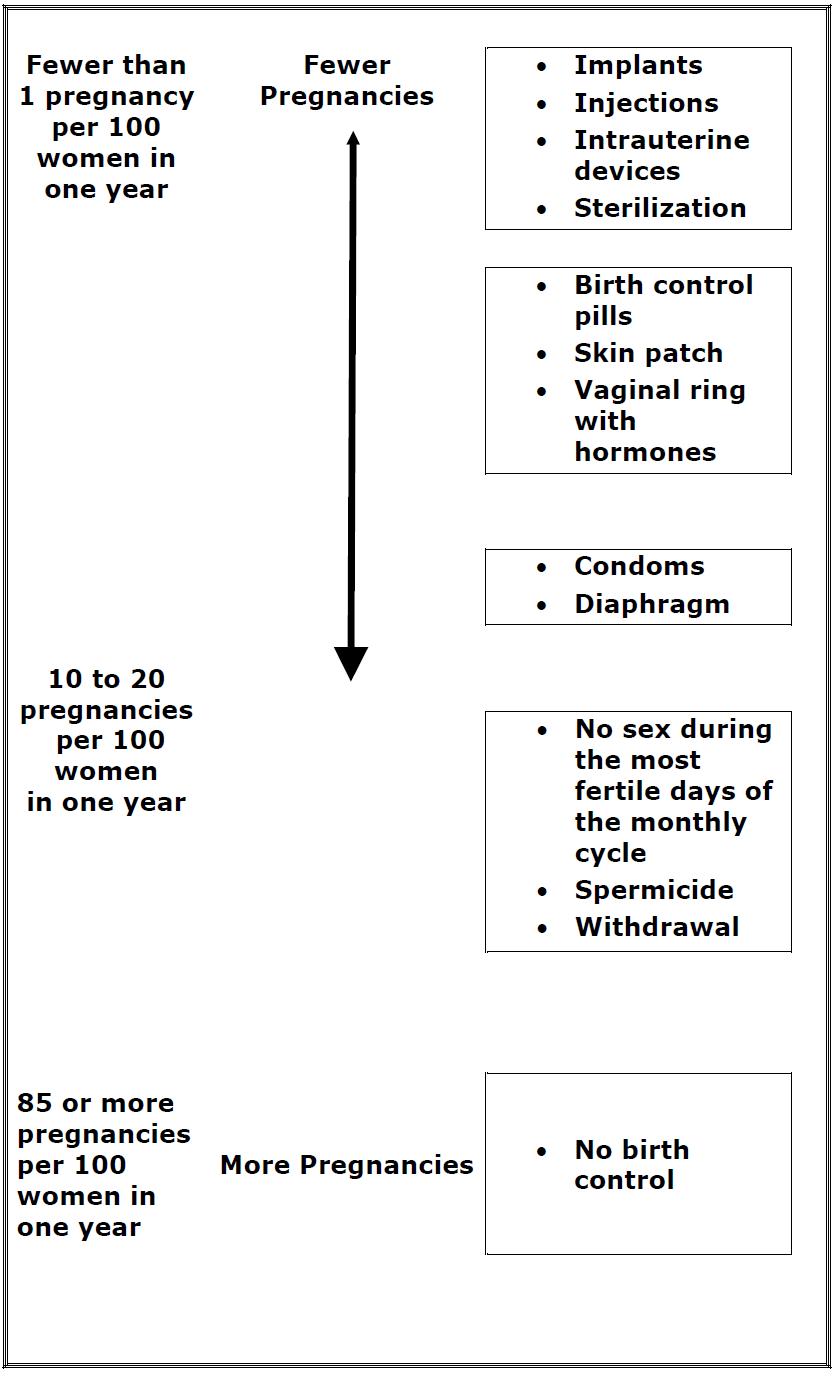
Patient InformationNexestaTM Fe (neks-ey-stuh Fe) (norethindrone and ethinyl estradiol tablets USP (chewable) and ferrous fumarate tablets (chewable)) What is the most important information I should know ...
-
Instructions For UseNexestaTM Fe (neks-ey-stuh Fe) (norethindrone and ethinyl estradiol tablets USP (chewable) and ferrous fumarate tablets (chewable)) Important Information about taking Nexesta Fe - Take 1 pill ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.4 mg/0.035 mg 1 Pouch LabelNDC 65862-926-87 - NexestaTM Fe - (Norethindrone and Ethinyl Estradiol - Tablets USP (Chewable) 0.4 mg/0.035 mg - and Ferrous Fumarate Tablets (Chewable)) Each of the 21 WHITE TO OFF-WHITE ...
-
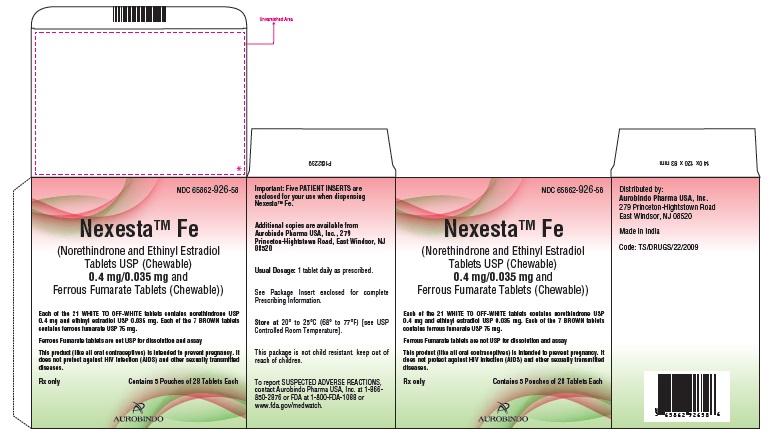
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.4 mg/0.035 mg Pouch CartonNDC 65862-926-58 - NexestaTM Fe - (Norethindrone and Ethinyl Estradiol Tablets USP (Chewable) 0.4 mg/0.035 mg - and Ferrous Fumarate Tablets (Chewable)) Each of the 21 WHITE TO OFF-WHITE ...
-
INGREDIENTS AND APPEARANCEProduct Information