Label: NEURACEQ- florbetaben f 18 injection, solution
- NDC Code(s): 54828-001-30, 54828-001-50
- Packager: Life Molecular Imaging, Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NEURACEQ safely and effectively. See full prescribing information for NEURACEQ. NEURACEQ (florbetaben F 18 injection), for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENeuraceq is indicated for Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated ...
-
2 DOSAGE AND ADMINISTRATION2.1 Radiation Safety - Drug Handling - Neuraceq is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration [ see ...
-
3 DOSAGE FORMS AND STRENGTHSNeuraceq is available in 50 mL multi-dose vials containing a clear solution at a strength of 50 MBq/mL to 5,000 MBq/mL (1.4 mCi/mL to 135 mCi/mL) florbetaben F18 at end of synthesis. At time of ...
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Risk for Image Misinterpretation and Other Errors - Errors may occur in the Neuraceq estimation of brain neuritic β-amyloid plaque density during image interpretation [ see Clinical ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSDrug-drug interaction studies have not been performed in patients to establish the extent, if any, to which concomitant medications may alter Neuraceq image results.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on Neuraceq use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or ...
-
10 OVERDOSAGEA pharmacological overdose of Neuraceq is unlikely given the relatively low doses used for diagnostic purposes. In the event of administration of a radiation overdose with Neuraceq, the absorbed ...
-
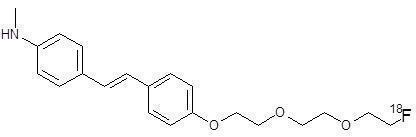
11 DESCRIPTIONNeuraceq contains florbetaben F18, a molecular imaging agent that binds to β-amyloid plaques in the brain, and is intended for use with PET imaging. Chemically, florbetaben F18 is described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Florbetaben F18 is a F18-labeled stilbene derivative, which binds to β-amyloid plaques in the brain. The F 18 isotope produces a positron signal that is detected by a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been performed to evaluate the carcinogenic potential of florbetaben. Florbetaben did not demonstrate mutagenic ...
-
14 CLINICAL STUDIESNeuraceq (doses ranging from 240 MBq to 360 MBq) was evaluated in three single arm clinical studies (Study A-C) that examined images from adults with a range of cognitive function, including some ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Neuraceq is supplied in a 50 mL glass vial containing up to 50 mL of a clear solution at a strength of 50 MBq/mL to 5,000 MBq/mL (1.4 mCi/mL to 135 mCi/mL) florbetaben F18 at ...
-
17 PATIENT COUNSELING INFORMATION• Instruct patients to inform their physician or healthcare provider if they are pregnant or breastfeeding. • Inform patients who are breastfeeding to use alternate infant nutrition sources (e.g ...
-
SPL UNCLASSIFIED SECTIONManufactured for Life Molecular Imaging Ltd., 25 Barnes Wallis Road, Fareham, Hampshire, PO15 5TT. United Kingdom. Neuraceq is a registered trademark of Life Molecular Imaging.
-
Neuraceq 50 mL Vial Label

-
Neuraceq Shield Label for 50 mL Vial

-
INGREDIENTS AND APPEARANCEProduct Information