Label: NEOPROFEN- ibuprofen lysine solution
- NDC Code(s): 55292-122-52
- Packager: Recordati Rare Diseases Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NEOPROFEN safely and effectively. See full prescribing information for NEOPROFEN. NEOPROFEN® (ibuprofen lysine) Injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENeoProfen is indicated to close a clinically significant patent ductus arteriosus (PDA) in premature infants weighing between 500 and 1500 g, who are no more than 32 weeks gestational age when ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose - A course of therapy is three doses of NeoProfen administered intravenously (administration via an umbilical arterial line has not been evaluated). An initial dose of 10 mg ...
-
3 DOSAGE FORMS AND STRENGTHS20 mg/2 mL (10 mg/mL) as a clear sterile preservative-free solution of the L-lysine salt of ibuprofen in a 2 mL single-use vial.
-
4 CONTRAINDICATIONSNeoProfen is contraindicated in: Preterm infants with proven or suspected infection that is untreated; Preterm infants with congenital heart disease in whom patency of the PDA is necessary for ...
-
5 WARNINGS AND PRECAUTIONS5.1 General - There are no long-term evaluations of the infants treated with ibuprofen at durations greater than the 36 weeks post-conceptual age observation period. Ibuprofen's effects on ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - The most frequently reported adverse events with NeoProfen were as shown in Table 1. Table 1. Adverse Events within 30 Days of Therapy in the Multicenter ...
-
7 DRUG INTERACTIONSDiuretics: Ibuprofen may reduce the effect of diuretics; diuretics can increase the risk of nephrotoxicity of NSAIDs in dehydrated patients. Monitor renal function in patients receiving ...
-
8 USE IN SPECIFIC POPULATIONS8.4 Pediatric Use - Safety and effectiveness have only been established in premature infants.
-
10 OVERDOSAGEThe following signs and symptoms have occurred in individuals (not necessarily in premature infants) following an overdose of oral ibuprofen: breathing difficulties, coma, drowsiness, irregular ...
-
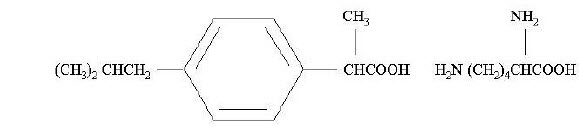
11 DESCRIPTIONNeoProfen® is a clear sterile preservative-free solution of the L-lysine salt of (±)-ibuprofen which is the active ingredient. (±)-Ibuprofen is a nonsteroidal anti-inflammatory agent (NSAID) ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action through which ibuprofen causes closure of a patent ductus arteriosus (PDA) in neonates is not known. In adults, ibuprofen is an inhibitor of ...
-
14 CLINICAL STUDIESIn a double-blind, multicenter clinical study premature infants of birth weight between 500 and 1000 g, less than 30 weeks post-conceptional age, and with echocardiographic evidence of a PDA were ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - NeoProfen (ibuprofen lysine) Injection is dispensed in treated1 clear glass single-use vials, each containing 2 mL of sterile solution (NDC 55292-122-52). The solution is not ...
-
17 PATIENT COUNSELING INFORMATIONInfection - NeoProfen may alter signs of infection. Patients' caregivers should be informed to monitor the infant for any signs of infection. Platelet Aggregation - Patients' caregivers ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Alcami Carolinas Corporation, Charleston, SC 29405, U.S.A. For: Recordati Rare Diseases Inc., Bridgewater, NJ 08807, U.S.A. ® Trademark of Recordati Rare Diseases Inc. Revised ...
-
PRINCIPAL DISPLAY PANEL - 2 mL Vial CartonNDC 55292-122-52 - 3 x 2 mL Single Dose Vials - NeoProfen® (ibuprofen lysine) injection - 20mg/2mL - (10mg/mL) Sterile Solution for - Intravenous Use Only - RECORDATI - RARE DISEASES - GROUP - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information