Label: PORTRAZZA- necitumumab solution
- NDC Code(s): 0002-7716-01
- Packager: Eli Lilly and Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PORTRAZZA safely and effectively. See full prescribing information for PORTRAZZA. PORTRAZZA (necitumumab) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CARDIOPULMONARY ARREST and HYPOMAGNESEMIA
- Cardiopulmonary arrest and/or sudden death occurred in 3.0% of patients treated with PORTRAZZA in combination with gemcitabine and cisplatin. Closely monitor serum electrolytes, including serum magnesium, potassium, and calcium, with aggressive replacement when warranted during and after PORTRAZZA administration [see Warnings and Precautions (5.1, 5.2)].
- Hypomagnesemia occurred in 83% of patients receiving PORTRAZZA in combination with gemcitabine and cisplatin, and was severe in 20% of patients. Monitor patients for hypomagnesemia, hypocalcemia, and hypokalemia prior to each dose of PORTRAZZA during treatment and for at least 8 weeks following completion of PORTRAZZA. Withhold PORTRAZZA for Grade 3 or 4 electrolyte abnormalities. Replete electrolytes as medically appropriate [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
1.1 Squamous Non-Small Cell Lung Cancer (NSCLC) PORTRAZZA™ is indicated, in combination with gemcitabine and cisplatin, for first-line treatment of patients with metastatic squamous non-small ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose and Schedule - The recommended dose of PORTRAZZA is 800 mg administered as an intravenous infusion over 60 minutes on Days 1 and 8 of each 3-week cycle prior to gemcitabine ...
-
3 DOSAGE FORMS AND STRENGTHS
Injection: 800 mg/50 mL (16 mg/mL) solution in a single-dose vial
-
4 CONTRAINDICATIONS
None
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiopulmonary Arrest - Cardiopulmonary arrest or sudden death occurred in 15 (3%) of 538 patients treated with PORTRAZZA plus gemcitabine and cisplatin as compared to 3 (0.6%) of 541 ...
-
6 ADVERSE REACTIONS
The following adverse drug reactions are discussed in greater detail in other sections of the label: Cardiopulmonary Arrest [see Boxed Warning and Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Based on animal data and its mechanism of action, PORTRAZZA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)] ...
-
10 OVERDOSAGE
There has been limited experience with PORTRAZZA overdose in human clinical trials. The highest dose of PORTRAZZA studied clinically in a human dose-escalation Phase 1 study was 1000 mg once a ...
-
11 DESCRIPTION
Necitumumab is an anti-EGFR recombinant human monoclonal antibody of the IgG1 kappa isotype that specifically binds to the ligand binding site of the human EGFR. Necitumumab has an approximate ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Necitumumab is a recombinant human lgG1 monoclonal antibody that binds to the human epidermal growth factor receptor (EGFR) and blocks the binding of EGFR to its ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been performed to assess the potential of necitumumab for carcinogenicity or genotoxicity. Fertility studies have not ...
-
14 CLINICAL STUDIES
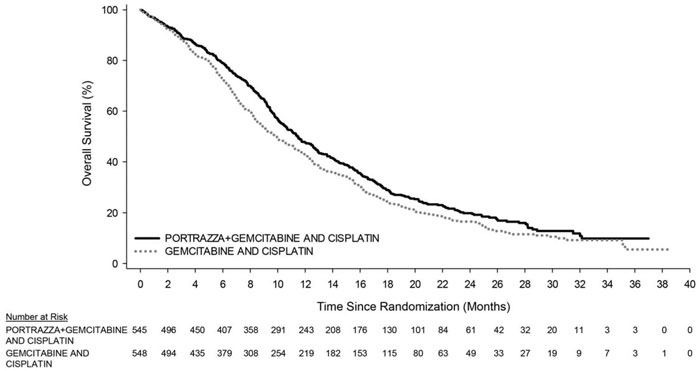
14.1 Squamous Non-Small Cell Lung Cancer - Study 1 was a randomized, multi-center open-label, controlled trial conducted in 1093 patients receiving gemcitabine and cisplatin first-line ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - PORTRAZZA is supplied in single-dose vials as a sterile, preservative-free solution: 800 mg/50 mL (16 mg/mL) NDC 0002-7716-01 - 16.2 Storage and ...
-
17 PATIENT COUNSELING INFORMATION
Hypomagnesemia - Advise patients of risk of decreased blood levels of magnesium, potassium and calcium. Take medicines to replace the electrolytes exactly as advised by the physician. [see Boxed ...
-
PORTRAZZA 800mg Single Dose VialRx only - NDC 0002-7716-01 - Portrazza™ (necitumumab) injection - 800 mg/50 mL - (16 mg/mL) For Intravenous Infusion Only - Must Dilute Prior to Use - Single-Dose Vial - Discard Unused Portion - Keep ...
-
INGREDIENTS AND APPEARANCEProduct Information