Patient Information for Head Lice Treatment
-
NATROBA™ (Nah-TRO-buh)
(spinosad)
topical suspension
-
Important: For use on scalp hair and scalp only. Do not get NATROBA in your eyes, mouth ...
Patient Information for Head Lice Treatment
NATROBA™ (Nah-TRO-buh)
(spinosad)
topical suspension
Important: For use on scalp hair and scalp only. Do not get NATROBA in your eyes, mouth, or vagina.
What is NATROBA?
NATROBA is a prescription medicine used to treat head lice on the scalp and hair of adults and children 6 months of age and older.
It is not known if NATROBA is safe and effective for children under 6 months of age.
See “How do I stop the spread of lice?” at the end of this leaflet for additional information on ways to stop the spread of lice.
Before you use NATROBA, tell your healthcare provider about all of your medical conditions, including if you or your child:
- have any skin conditions or sensitivities
- are pregnant or plan to become pregnant. It is not known if NATROBA can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Talk to your healthcare provider about the best way to feed your baby during treatment with NATROBA.
How should I use NATROBA?
-
See the detailed “Instructions for Use” at the end of this leaflet.
- Apply NATROBA exactly as prescribed by your healthcare provider. Your healthcare provider will prescribe the treatment that is right for you. Do not change your treatment unless you talk to your healthcare provider.
- Apply NATROBA when your hair is dry. Do not wet your hair before applying NATROBA.
- It is important to apply enough NATROBA to completely coat all of your hair and scalp. Leave NATROBA on your hair and scalp for a full 10 minutes.
- If live lice are seen one week (7 days) after you applied NATROBA, you will need to apply NATROBA again.
- Because you need to completely cover all of the lice with NATROBA, you may need help in applying NATROBA to your scalp and hair.
- Children will need an adult to apply NATROBA for them.
-
Do not get NATROBA into your eyes. If NATROBA gets in your eye, rinse well with water right away.
- If you have any scalp irritation after you apply NATROBA, call your healthcare provider right away.
- Wash your hands after you apply NATROBA.
-
Do not swallow NATROBA. If swallowed, call Poison Control at 1-800-222-1222 or go to the nearest emergency room right away.
What are the possible side effects of NATROBA?
The most common side effects of NATROBA include redness where NATROBA is applied and redness to eyes.
These are not all the possible side effects of NATROBA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NATROBA?
- Store NATROBA at room temperature between 68°F to 77°F (20°C to 25°C).
Keep NATROBA and all medicines out of the reach of children.
General information about the safe and effective use of NATROBA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NATROBA for a condition for which it was not prescribed. Do not give NATROBA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NATROBA that is written for health professionals.
What are the ingredients in NATROBA?
Active ingredient: spinosad
Inactive ingredients: Benzyl Alcohol, Butylated Hydroxytoluene, Ceteareth-20, Cetearyl Alcohol, FD&C Yellow #6, Hexylene Glycol, Hydroxyethyl Cellulose, Isopropyl Alcohol, Propylene Glycol, Stearalkonium Chloride, Water, Hydrochloric acid (HCl) as pH adjuster.
Instructions for Use
Before you use NATROBA, it is important that you read this Instructions for Use. Be sure that you read, understand, and follow this Instructions for Use so that you use NATROBA the right way. Ask your healthcare provider or pharmacist if you have questions about the right way to use NATROBA.
Important information:
- Your hair and scalp must be dry before applying NATROBA.
- For very thick, medium length hair or long hair, an entire bottle (120mL) of NATROBA may be needed to cover the scalp and hair. Less NATROBA may be needed for shorter, thinner hair.
How to apply NATROBA to your scalp and hair:
Step 1
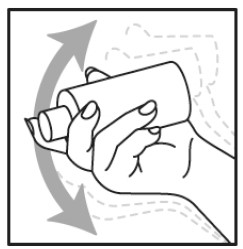
- Shake NATROBA bottle well right before use.
Step 2
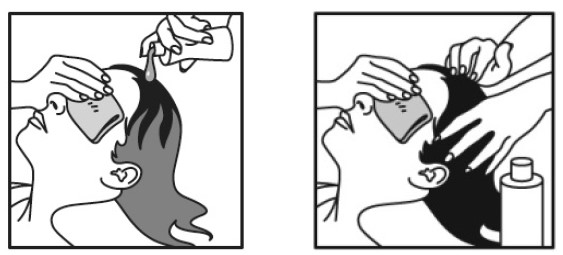
- Cover your face and eyes with a towel and keep your eyes closed tightly.
- Apply NATROBA directly to dry hair and scalp.
- Completely cover the scalp and hair closest to the scalp first, and then apply outwards towards the ends of the hair.
- It is important to apply enough NATROBA to cover your entire scalp and hair so that all lice and eggs are exposed to NATROBA.
Step 3
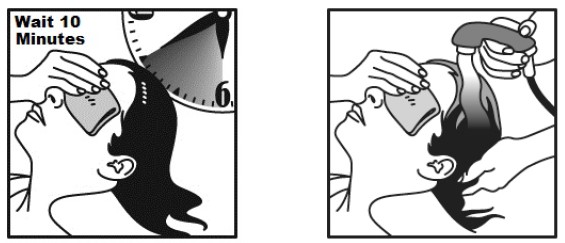
- Allow NATROBA to stay on your scalp and hair for 10 minutes. Use a timer or clock and start timing after you have completely covered your hair and scalp with NATROBA.
- Continue to keep eyes covered to prevent dripping into your eyes.
- After 10 minutes, completely rinse NATROBA from your hair and scalp with warm water.
- You may use a fine-tooth comb to remove treated lice and nits from the hair and scalp, but combing is not required.
-
Wash your hands after applying NATROBA.
- It is okay to shampoo your hair any time after the treatment.
If you see live lice on your scalp or hair one week (7 days), after your first treatment, repeat the steps above.
How do I stop the spread of lice?
To help prevent the spread of lice from one person to another, here are some steps you can take:
- Avoid direct head-to-head contact with anyone known to have live, crawling lice.
- Do not share combs, brushes, hats, scarves, bandannas, ribbons, barrettes, hair bands, towels, helmets, or other hair-related personal items with anyone else, whether they have lice or not.
- Avoid sleepovers and slumber parties during lice outbreaks. Lice can live in bedding, pillows, and carpets that have recently been used by someone with lice.
- After finishing treatment with lice medicine, check everyone in your family for lice after one week. Be sure to talk to your healthcare provider about treatments for those who have lice.
- Machine-wash or dry-clean any bedding, towels and clothing used by anyone having lice. Machine-wash at high temperatures (150°F) and tumble in a hot dryer for 20 minutes.
- Wash personal items such as combs, brushes, and hair clips in hot water.
Manufactured for: ParaPRO LLC, Carmel, IN 46032
Distributed by: ParaPRO LLC, Brownsburg, IN 46112
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Revised: 04/2021
Patient Information for Scabies Treatment
NATROBA™ (Nah-TRO-buh)
(spinosad)
topical suspension
Important: For use on skin only. Do not get NATROBA in your eyes, mouth, or vagina.
What is NATROBA?
NATROBA is a prescription medicine used to treat scabies in adults and children 4 years of age and older.
It is not known if NATROBA is safe and effective in children under 4 years of age.
See “How do I stop the spread of scabies?” at the end of this leaflet.
Before you use NATROBA, tell your healthcare provider about all of your medical conditions, including if you or your child:
- have any skin conditions or sensitivities
- are pregnant or plan to become pregnant. It is not known if NATROBA can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Wash the breast area with soap and water to remove NATROBA before breastfeeding to avoid exposure to your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with NATROBA.
How should I use NATROBA?
-
See the detailed "Instructions for Use" at the end of this leaflet.
- Apply NATROBA exactly as prescribed by your healthcare provider. Your healthcare provider will prescribe the treatment that is right for you. Do not change your treatment unless you talk to your healthcare provider.
- It is important to apply enough NATROBA to completely cover your body from your neck to the soles of your feet. Make sure you apply to folds of skin, between fingers and toes, and under finger and toe nails.
- If you are balding, apply NATROBA to the scalp, hairline, temples, and forehead.
- Wait a full 10 minutes to allow NATROBA to soak into the skin and dry before getting dressed. Leave NATROBA on the skin for at least 6 hours before showering or bathing.
- Because you need to completely cover your body from the neck down with NATROBA, you may need help in applying NATROBA to ensure full coverage.
- Children will need an adult to apply NATROBA for them.
-
Do not get NATROBA into your eyes. If NATROBA gets in your eye, rinse well with water right away.
- If you have any skin irritation after you apply NATROBA, call your healthcare provider right away.
- Wash your hands after applying NATROBA to someone else.
-
Do not swallow NATROBA. If swallowed, call Poison Control at 1-800-222-1222 or go to the nearest emergency room right away.
What are the possible side effects of NATROBA?
The most common side effects of NATROBA include irritation (including pain and burning) at application sites and dry skin.
These are not all the possible side effects of NATROBA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NATROBA?
- Store NATROBA at room temperature between 68°F to 77°F (20°C to 25°C).
Keep NATROBA and all medicines out of the reach of children.
General information about the safe and effective use of NATROBA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NATROBA for a condition for which it was not prescribed. Do not give NATROBA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NATROBA that is written for health professionals.
What are the ingredients in NATROBA?
Active ingredient: spinosad
Inactive ingredients: Benzyl Alcohol, Butylated Hydroxytoluene, Ceteareth-20, Cetearyl Alcohol, FD&C Yellow #6, Hexylene Glycol, Hydroxyethyl Cellulose, Isopropyl Alcohol, Propylene Glycol, Stearalkonium Chloride, Water, Hydrochloric acid (HCl) as pH adjuster.
Instructions for Use
Before you use NATROBA, it is important that you read this Instructions for Use. Be sure that you read, understand, and follow this Instructions for Use so that you use NATROBA the right way. Ask your healthcare provider or pharmacist if you have questions about the right way to use NATROBA.
How to apply NATROBA to your body:
Step 1
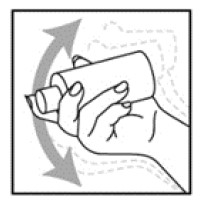
- Shake the NATROBA bottle well right before use
Step 2
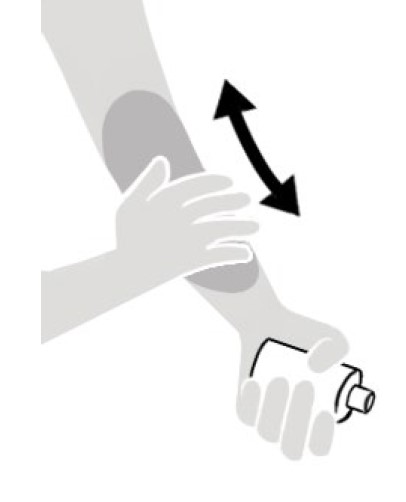
Figure A
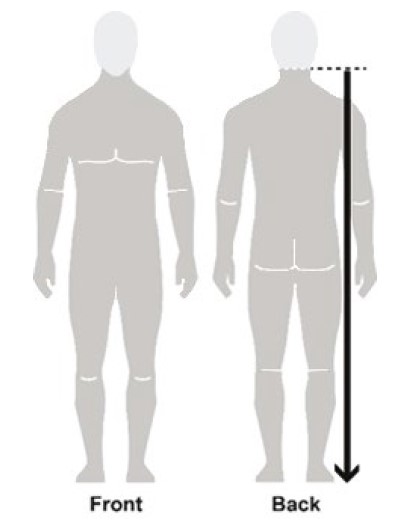
Figure B
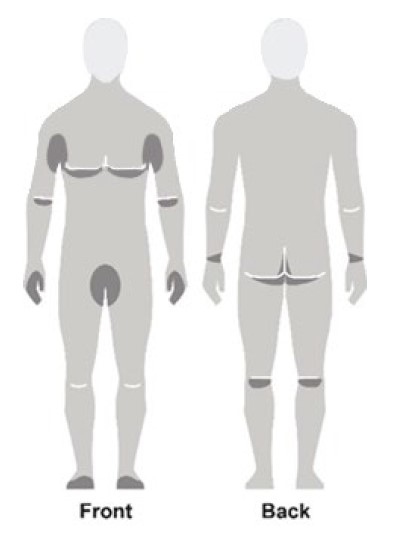
Figure C
- Apply NATROBA to the skin. (See Figure A)
- Apply enough NATROBA to completely cover your body from your neck to the soles of your feet. (See Figure B). Make sure you apply to folds of skin, between fingers and toes, and under finger and toe nails. (See Figure C)
- If you are balding, you should also apply NATROBA to the scalp, hairline, temples and forehead.
- You may need help applying NATROBA to ensure full coverage.
- If you do not completely cover your body with NATROBA, some scabies mites may escape treatment.
- Wash your hands after applying NATROBA to someone else.
Step 3
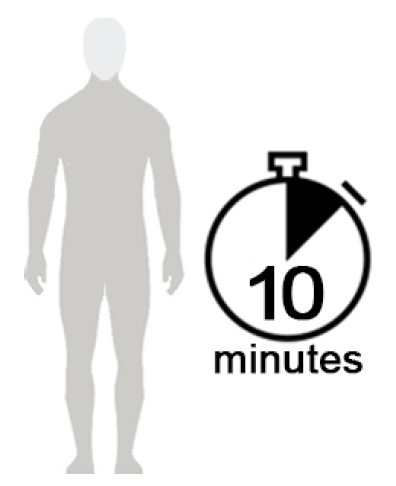
Figure D
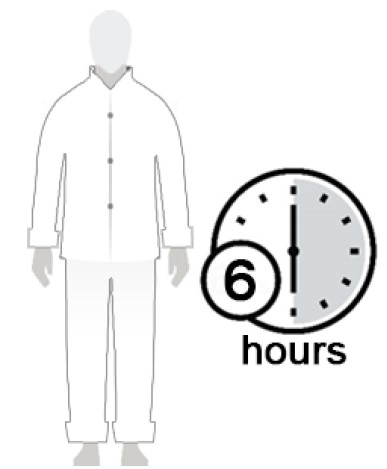
Figure E
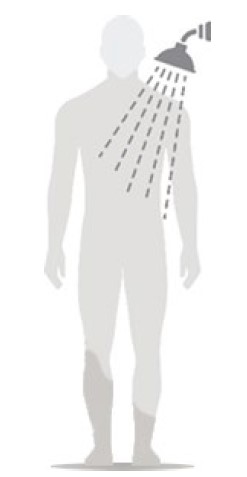
Figure F
- Allow NATROBA to soak into the skin and dry for 10 minutes. Use a timer or clock and start timing after you have completely covered your body with NATROBA. (See Figure D)
- After 10 minutes, you can get dressed. (See Figure E)
- Wait at least 6 hours before showering or bathing. (See Figure F)
How do I stop the spread of scabies?
To help prevent the spread of scabies from one person to another, here are some steps you can take:
- Get household members checked by their healthcare provider.
- Avoid direct body-to-body contact with anyone known to have scabies, including sexual partners.
- Do not share clothing, towels, bedding or linens with anyone else. The scabies mite can live in bedding, pillows, and carpets that have recently been used by someone with scabies.
- Machine-wash or dry clean any bedding, clothing and towels that you have used anytime during the three days before treatment by washing in hot water and drying in a hot dryer, or by sealing in a plastic bag for at least 72 hours. Machine-wash at high temperatures (150°F) and tumble in a hot dryer for 20 minutes.
Manufactured for: ParaPRO LLC, Carmel, IN 46032
Distributed by: ParaPRO LLC, Brownsburg, IN 46112
NAT-PI-002
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Issued: 04/2021
Close