Label: SYNAREL- nafarelin acetate spray, metered
- NDC Code(s): 0025-0166-08
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONCENTRAL PRECOCIOUS PUBERTY (FOR ENDOMETRIOSIS, SEE REVERSE SIDE) PHYSICIAN LABELING
-
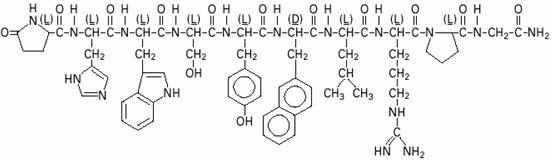
DESCRIPTIONSYNAREL (nafarelin acetate) Nasal Solution is intended for administration as a spray to the nasal mucosa. Nafarelin acetate, the active component of SYNAREL Nasal Solution, is a decapeptide with ...
-
CLINICAL PHARMACOLOGYNafarelin acetate is a potent agonistic analog of gonadotropin-releasing hormone (GnRH). At the onset of administration, nafarelin stimulates the release of the pituitary gonadotropins, LH and ...
-
INDICATIONS AND USAGE FOR CENTRAL PRECOCIOUS PUBERTY(For Endometriosis, See Reverse Side) SYNAREL is indicated for treatment of central precocious puberty (CPP) (gonadotropin-dependent precocious puberty) in children of both sexes. The diagnosis of ...
-
CONTRAINDICATIONS1. Hypersensitivity to GnRH, GnRH agonist analogs or any of the excipients in SYNAREL; 2. Undiagnosed abnormal vaginal bleeding; 3. Use in pregnancy or in women who may become pregnant while ...
-
WARNINGSThe diagnosis of central precocious puberty (CPP) must be established before treatment is initiated. Regular monitoring of CPP patients is needed to assess both patient response as well as ...
-
PRECAUTIONSGeneral - As with other drugs that stimulate the release of gonadotropins or that induce ovulation, in adult women with endometriosis ovarian cysts have been reported to occur in the first two ...
-
ADVERSE REACTIONSIn clinical trials of 155 pediatric patients, 2.6% reported symptoms suggestive of drug sensitivity, such as shortness of breath, chest pain, urticaria, rash, and pruritus. In these 155 patients ...
-
OVERDOSAGEIn experimental animals, a single subcutaneous administration of up to 60 times the recommended human dose (on a µg/kg basis, not adjusted for bioavailability) had no adverse effects. At present ...
-
DOSAGE AND ADMINISTRATIONFor the treatment of central precocious puberty (CPP), the recommended daily dose of SYNAREL is 1600 µg. The dose can be increased to 1800 µg daily if adequate suppression cannot be achieved at ...
-
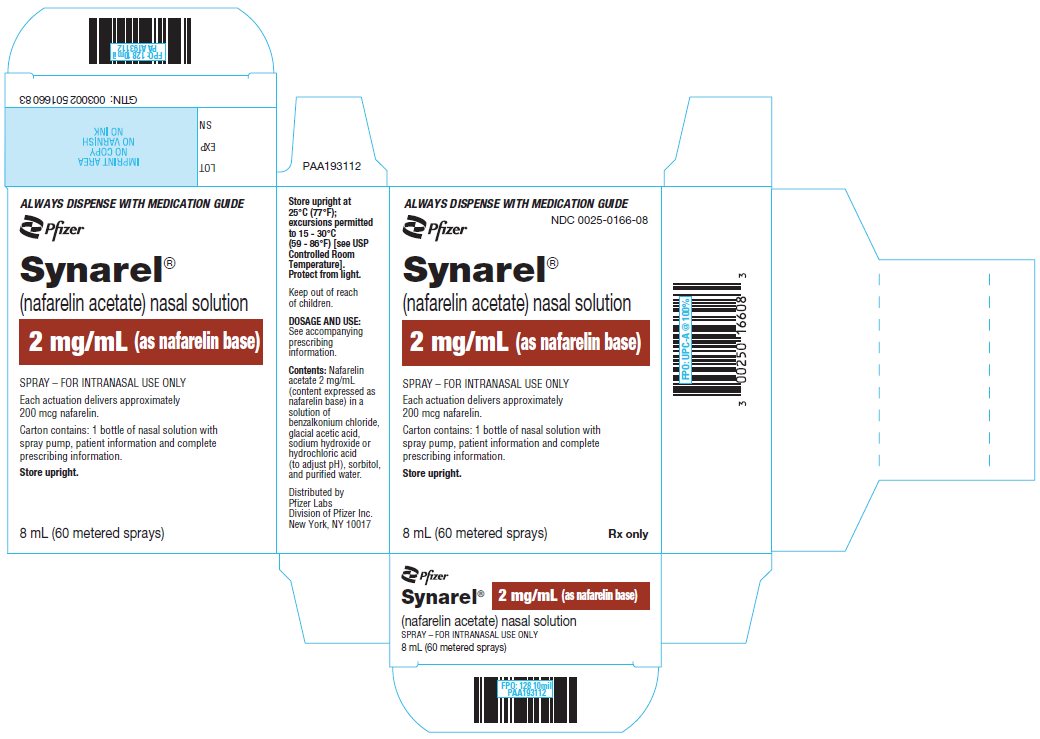
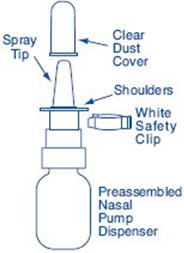
HOW SUPPLIEDEach 0.5 ounce bottle (NDC 0025-0166-08) contains 8 mL SYNAREL (nafarelin acetate) Nasal Solution 2 mg/mL (as nafarelin base), and is supplied with a metered spray pump that delivers 200 µg of ...
-
SPL UNCLASSIFIED SECTIONLAB-1048-4.0 - Revised: April 2022
-
SPL UNCLASSIFIED SECTIONSynarel® (nafarelin acetate) nasal solution - ENDOMETRIOSIS (FOR CENTRAL PRECOCIOUS PUBERTY, SEE REVERSE SIDE) PHYSICIAN LABELING
-
DESCRIPTIONSYNAREL (nafarelin acetate) Nasal Solution is intended for administration as a spray to the nasal mucosa. Nafarelin acetate, the active component of SYNAREL Nasal Solution, is a decapeptide with ...
-
CLINICAL PHARMACOLOGYNafarelin acetate is a potent agonistic analog of gonadotropin-releasing hormone (GnRH). At the onset of administration, nafarelin stimulates the release of the pituitary gonadotropins, LH and ...
-
INDICATIONS AND USAGE FOR ENDOMETRIOSIS(For Central Precocious Puberty, See Reverse Side) SYNAREL is indicated for management of endometriosis, including pain relief and reduction of endometriotic lesions. Experience with SYNAREL for ...
-
CONTRAINDICATIONS1. Hypersensitivity to GnRH, GnRH agonist analogs or any of the excipients in SYNAREL; 2. Undiagnosed abnormal vaginal bleeding; 3. Use in pregnancy or in women who may become pregnant while ...
-
WARNINGSSafe use of nafarelin acetate in pregnancy has not been established clinically. Before starting treatment with SYNAREL, pregnancy must be excluded. When used regularly at the recommended dose ...
-
PRECAUTIONSGeneral - As with other drugs that stimulate the release of gonadotropins or that induce ovulation, ovarian cysts have been reported to occur in the first two months of therapy with SYNAREL ...
-
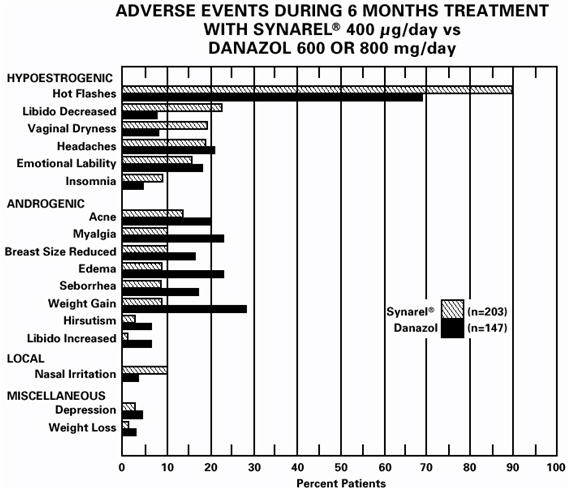
ADVERSE REACTIONSClinical Studies - In formal clinical trials of 1509 healthy adult patients, symptoms suggestive of drug sensitivity, such as shortness of breath, chest pain, urticaria, rash and pruritus ...
-
OVERDOSAGEIn experimental animals, a single subcutaneous administration of up to 60 times the recommended human dose (on a µg/kg basis, not adjusted for bioavailability) had no adverse effects. At present ...
-
DOSAGE AND ADMINISTRATIONFor the management of endometriosis, the recommended daily dose of SYNAREL is 400 µg. This is achieved by one spray (200 µg) into one nostril in the morning and one spray into the other nostril in ...
-
HOW SUPPLIEDEach 0.5 ounce bottle (NDC 0025-0166-08) contains 8 mL SYNAREL (nafarelin acetate) Nasal Solution 2 mg/mL (as nafarelin base), and is supplied with a metered spray pump that delivers 200 µg of ...
-
SPL UNCLASSIFIED SECTIONLAB-0173-13.0 - Revised: January 2023
-
PATIENT PACKAGE INSERTSYNAREL - nafarelin acetate - Nasal Spray - Patient Instructions for Use - Introduction - Your doctor has prescribed SYNAREL Nasal Solution to treat your symptoms of endometriosis. This pamphlet has ...
-
MEDICATION GUIDEMEDICATION GUIDE - SYNAREL (sin-na-rell) (nafarelin acetate) nasal solution - What is the most important information I should know about SYNAREL? • Some people taking GnRH agonists ...
-
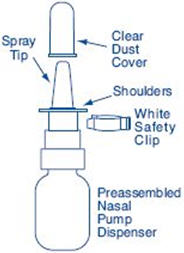
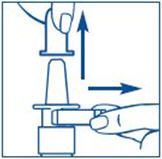
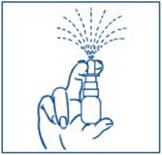
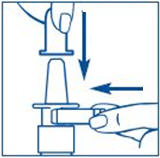
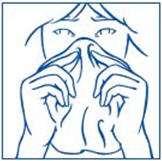
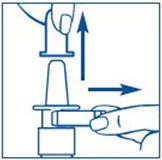
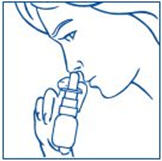
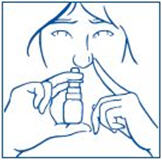
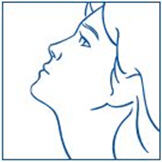
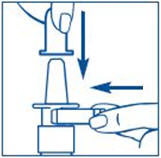
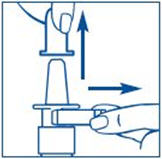
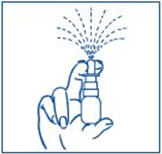
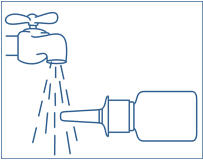
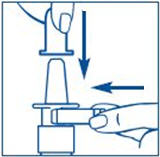
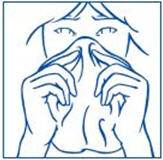
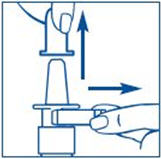
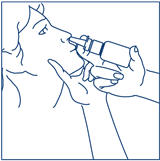
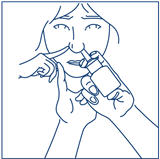
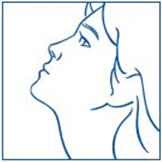
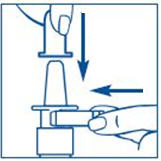
PATIENT PACKAGE INSERTInstructions for Use - SYNAREL (sin-na-rell) (nafarelin acetate) nasal solution - For use in the nose only. Figure A - Before you use SYNAREL nasal spray for the first time, you will ...
-
PRINCIPAL DISPLAY PANEL - 8 mL Bottle LabelALWAYS DISPENSE WITH MEDICATION GUIDE - Pfizer - NDC 0025-0166-08 - Synarel® (nafarelin acetate) nasal solution - 2 mg/mL (as nafarelin base) SPRAY - FOR INTRANASAL USE ONLY. Each actuation delivers ...
-
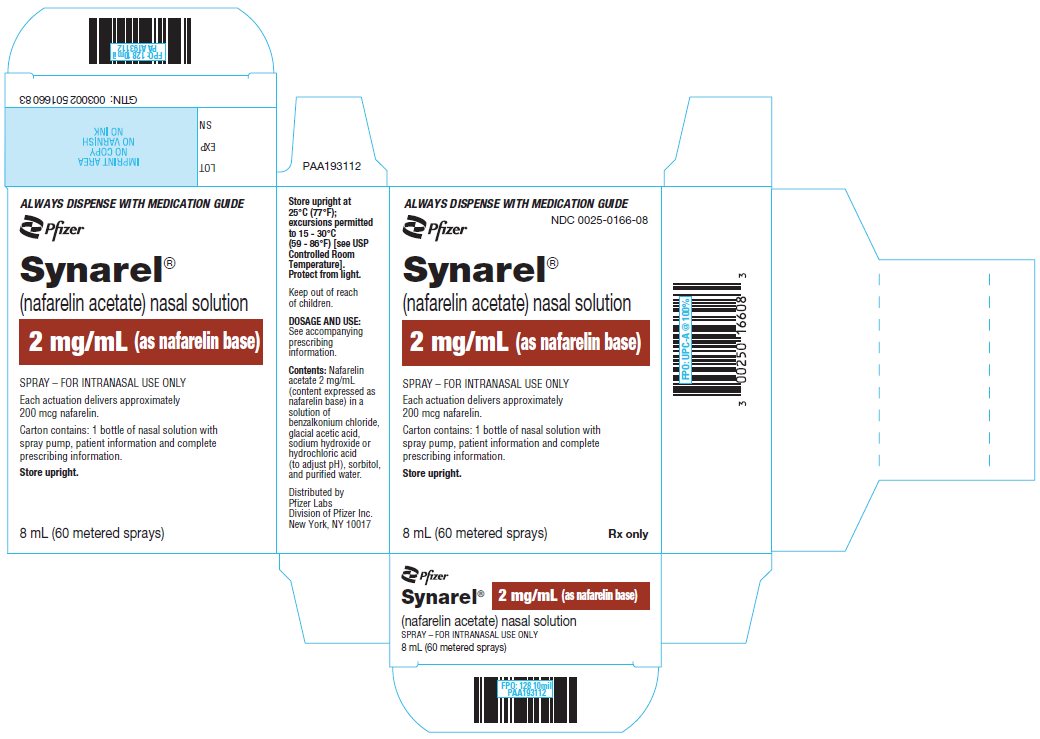
PRINCIPAL DISPLAY PANEL - 8 mL Bottle CartonALWAYS DISPENSE WITH MEDICATION GUIDE - Pfizer - NDC 0025-0166-08 - Synarel® (nafarelin acetate) nasal solution - 2 mg/mL (as nafarelin base) SPRAY – FOR INTRANASAL USE ONLY - Each actuation delivers ...
-
INGREDIENTS AND APPEARANCEProduct Information