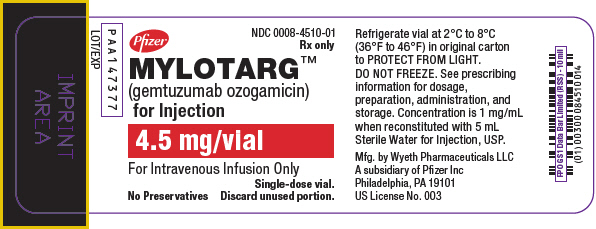
Label: MYLOTARG- gemtuzumab ozogamicin injection, powder, lyophilized, for solution
- NDC Code(s): 0008-4510-01
- Packager: Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 1, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MYLOTARG safely and effectively. See full prescribing information for MYLOTARG. MYLOTARG™ (gemtuzumab ozogamicin) for injection ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HEPATOTOXICITY
Hepatotoxicity, including severe or fatal hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), has been reported in association with the use of MYLOTARG as a single agent, and as part of a combination chemotherapy regimen. Monitor frequently for signs and symptoms of VOD after treatment with MYLOTARG. (5.1 and 6.1)
Close -
1 INDICATIONS AND USAGE1.1 Newly-Diagnosed CD33-positive Acute Myeloid Leukemia (AML) MYLOTARG is indicated for the treatment of newly-diagnosed CD33-positive acute myeloid leukemia in adults and pediatric patients 1 ...
-
2 DOSAGE AND ADMINISTRATION2.1 Premedication and Special Considerations - • Premedicate adults with acetaminophen 650 mg orally and diphenhydramine 50 mg orally or intravenously 1 hour prior to MYLOTARG dosing and 1 mg/kg ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 4.5 mg as a white to off-white lyophilized cake or powder in a single-dose vial for reconstitution and further dilution.
-
4 CONTRAINDICATIONSMYLOTARG is contraindicated in patients with a history of hypersensitivity to the active substance in MYLOTARG or any of its components or to any of the excipients. Reactions have included ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity, Including Veno-occlusive Liver Disease (VOD) Hepatotoxicity, including life-threatening and sometimes fatal hepatic VOD events, have been reported in patients receiving ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Hepatotoxicity, including VOD [see Warnings and Precautions (5.1)] • Infusion-related reactions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and findings from animal studies [see Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)], MYLOTARG can cause ...
-
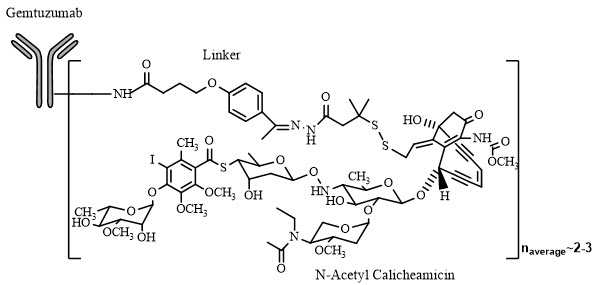
11 DESCRIPTIONGemtuzumab ozogamicin is an antibody-drug conjugate (ADC) composed of the CD33-directed monoclonal antibody (hP67.6; recombinant humanized immunoglobulin [Ig] G4, kappa antibody produced by ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Gemtuzumab ozogamicin is a CD33-directed antibody-drug conjugate (ADC). The antibody portion (hP67.6) recognizes human CD33 antigen. The small molecule, N-acetyl gamma ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Formal carcinogenicity studies have not been conducted with gemtuzumab ozogamicin. In toxicity studies, rats were dosed weekly for 6 ...
-
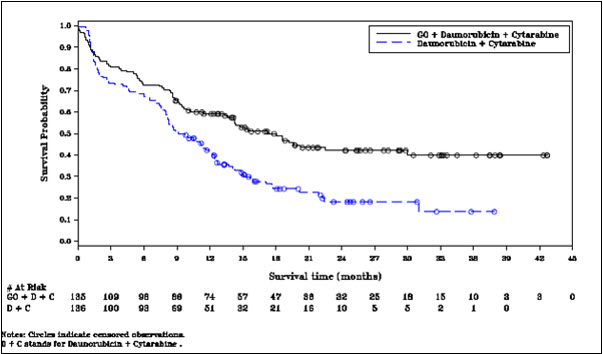
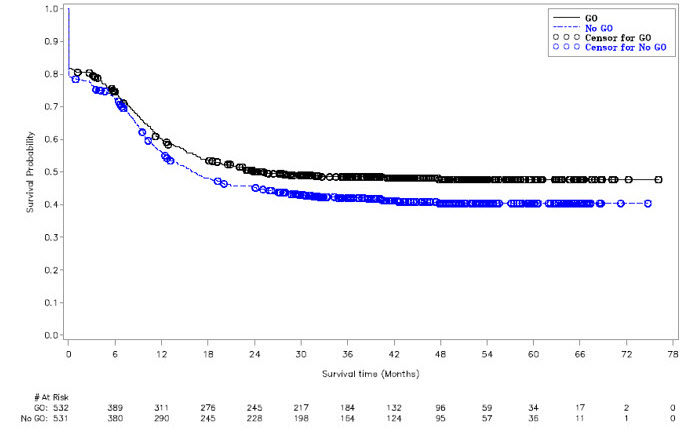
14 CLINICAL STUDIES14.1 Newly-Diagnosed CD33-positive AML - Study ALFA-0701 - MYLOTARG in combination with chemotherapy was evaluated in ALFA-0701 (NCT00927498), a multicenter, randomized, open-label Phase 3 ...
-
15 REFERENCES1. OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMYLOTARG (gemtuzumab ozogamicin) for injection is a white to off-white lyophilized cake or powder supplied in a carton (NDC 0008-4510-01) containing one 4.5 mg single-dose vial [see Dosage and ...
-
17 PATIENT COUNSELING INFORMATIONHepatotoxicity, Including Veno-occlusive Liver Disease (VOD) Inform patients that liver problems, including severe, life-threatening, or fatal VOD may develop during MYLOTARG treatment. Prior ...
-
SPL UNCLASSIFIED SECTIONThis product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com. US License No. 003 - LAB-0868-8.0
-
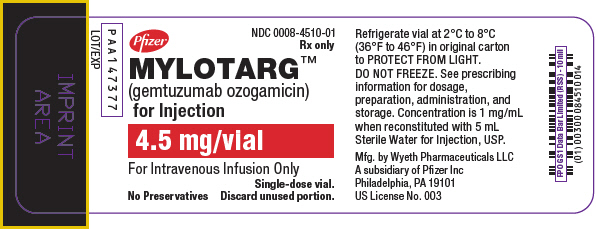
PRINCIPAL DISPLAY PANEL - 4.5 mg Vial LabelPfizer - NDC 0008-4510-01 - Rx only - MYLOTARG™ (gemtuzumab ozogamicin) for Injection - 4.5 mg/vial - For Intravenous Infusion Only - Single-dose vial. No Preservatives - Discard unused portion.
-
PRINCIPAL DISPLAY PANEL - 4.5 mg Vial CartonPfizer - NDC 0008-4510-01 - MYLOTARG™ (gemtuzumab ozogamicin) for Injection - 4.5 mg/vial - For Intravenous Infusion Only - Reconstitution and dilution - required - No Preservatives - One Single-Dose Vial ...
-
INGREDIENTS AND APPEARANCEProduct Information