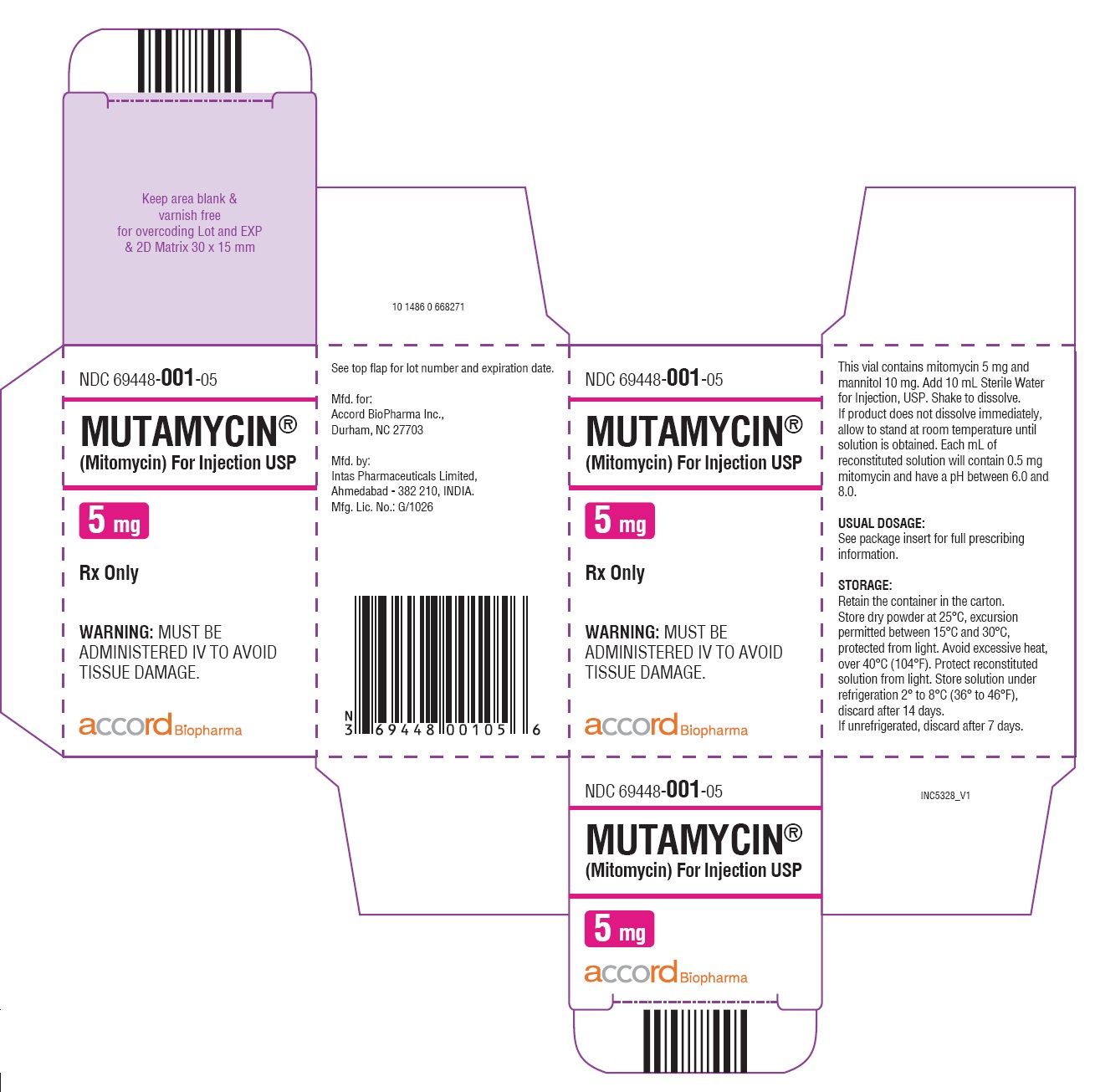
Label: MUTAMYCIN- mitomycin injection, powder, lyophilized, for solution
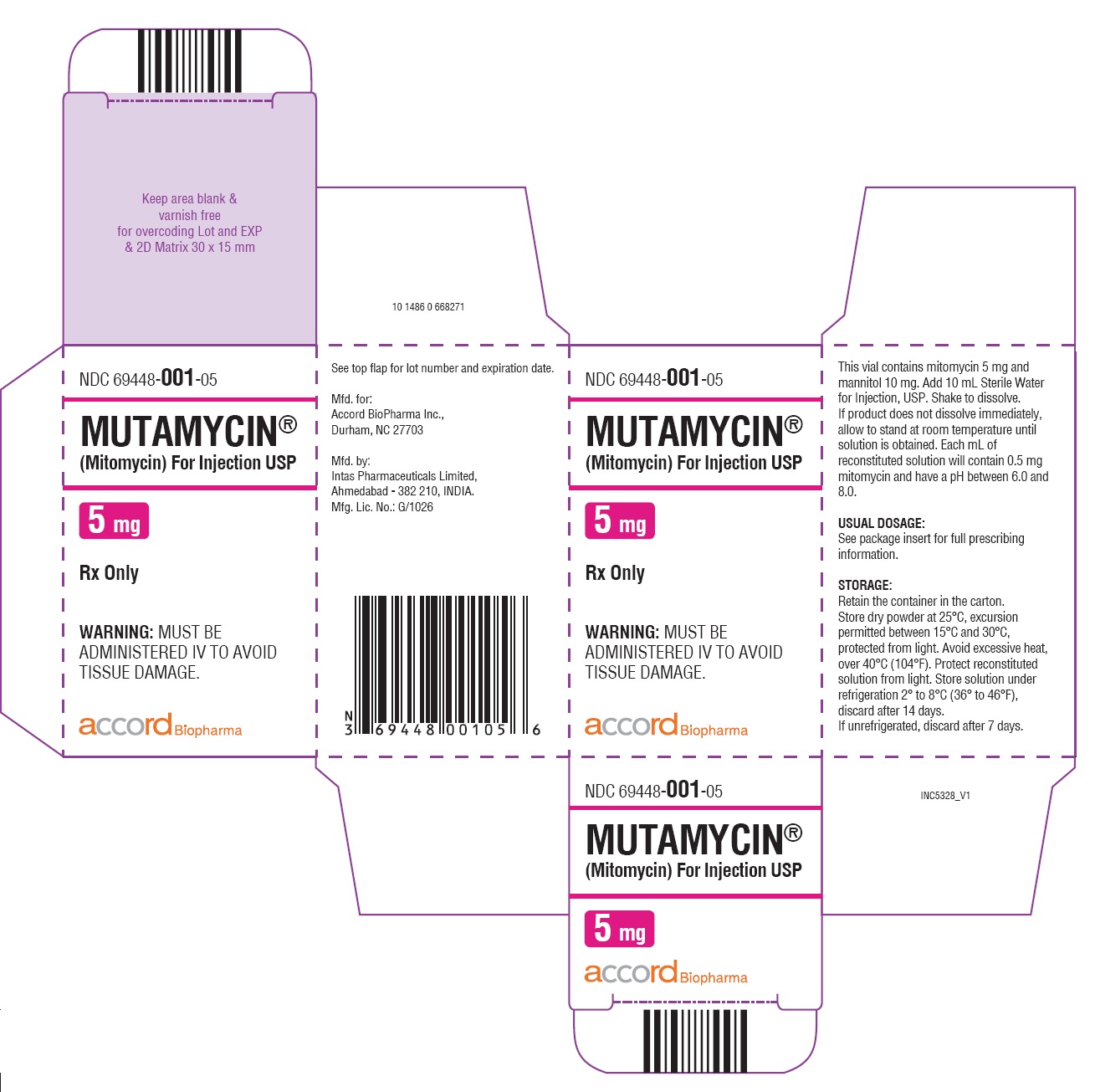
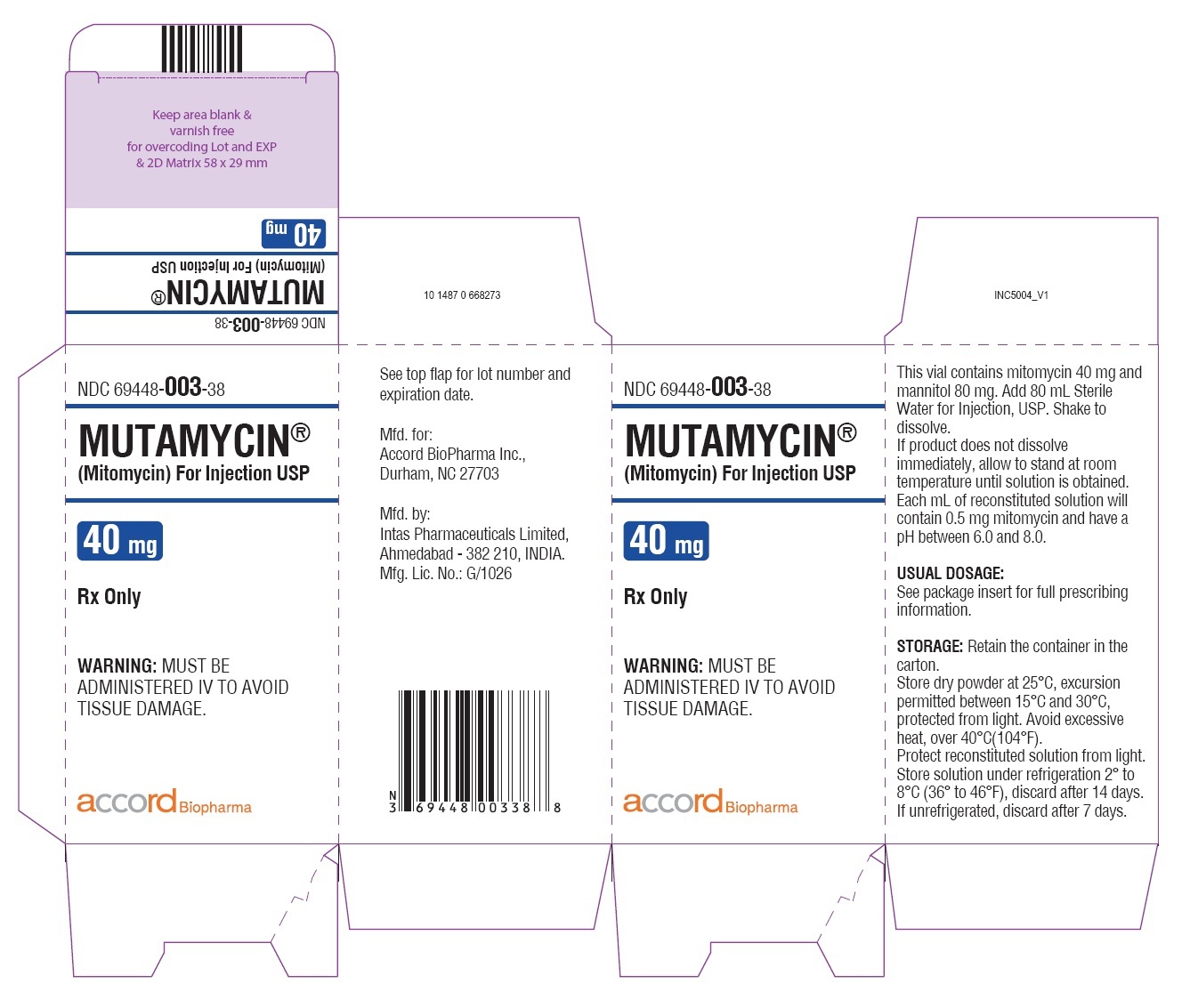
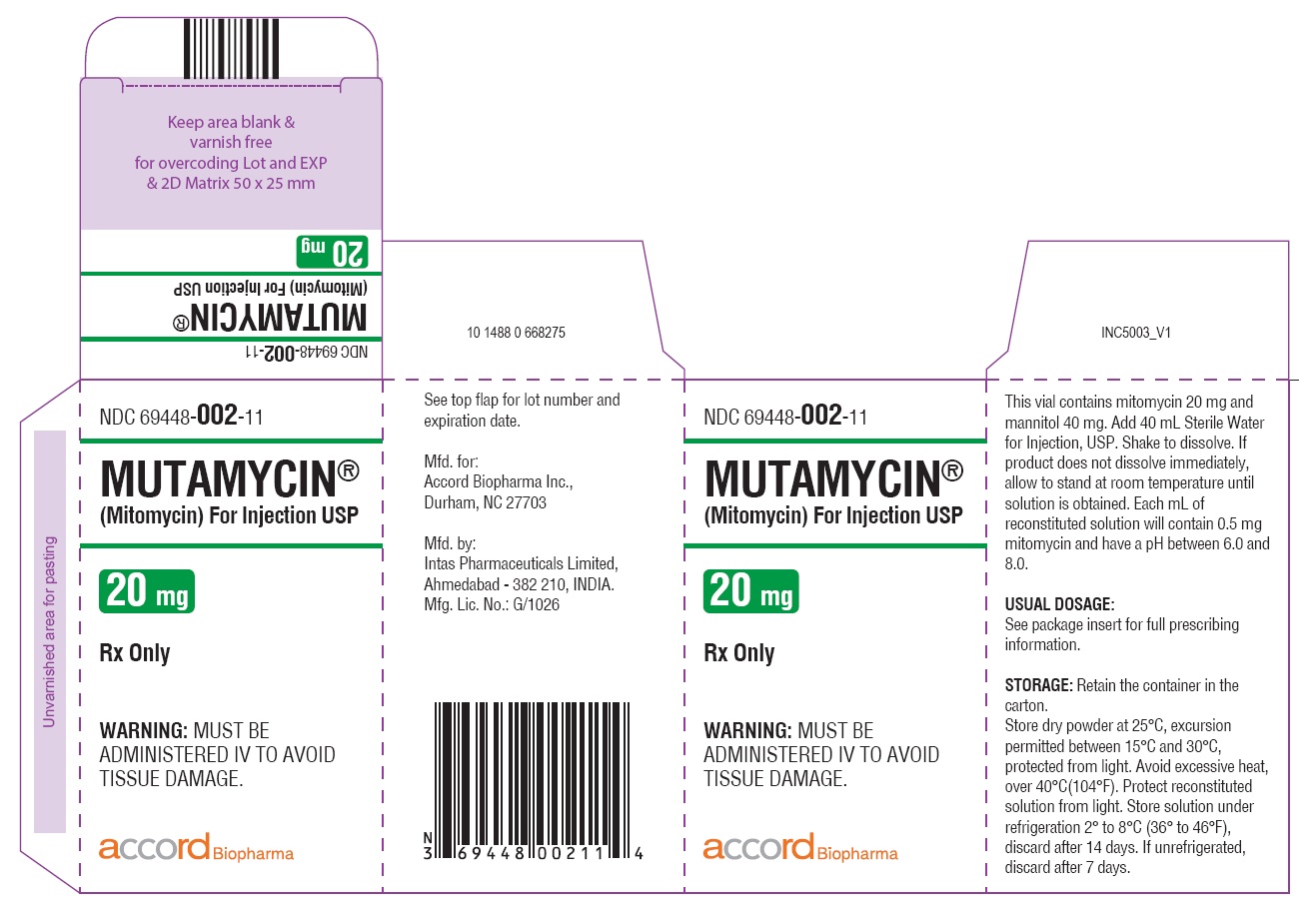
- NDC Code(s): 69448-001-05, 69448-002-11, 69448-003-38
- Packager: Accord BioPharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - WARNING - Mitomycin should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy ...
-
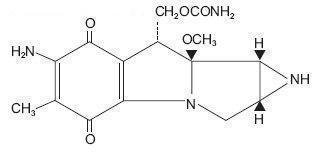
DESCRIPTIONMitomycin (also known as mitomycin and/or mitomycin-C) is an antibiotic isolated from the broth of Streptomyces caespitosus which has been shown to have antitumor activity. The compound is heat ...
-
CLINICAL PHARMACOLOGYMitomycin selectively inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking. At high concentrations ...
-
INDICATIONS AND USAGEMUTAMYCIN - ® (Mitomycin) for Injection is not recommended as single-agent, primary therapy. It has been shown to be useful in the therapy of disseminated adenocarcinoma of the stomach ...
-
CONTRAINDICATIONSMitomycin is contraindicated in patients who have demonstrated a hypersensitive or idiosyncratic reaction to it in the past. Mitomycin is contraindicated in patients with thrombocytopenia ...
-
WARNINGSPatients being treated with mitomycin must be observed carefully and frequently during and after therapy. The use of mitomycin results in a high incidence of bone marrow suppression, particularly ...
-
PRECAUTIONSAcute shortness of breath and severe bronchospasm have been reported following the administration of vinca alkaloids in patients who had previously or simultaneously received mitomycin. The onset ...
-
Geriatric UseInsufficient data from clinical studies of mitomycin are available for patients 65 years of age and older to determine whether they respond differently than younger patients. Postmarketing ...
-
ADVERSE REACTIONSBone Marrow Toxicity - This was the most common and most serious toxicity, occurring in 605 of 937 patients (64.4%). Thrombocytopenia and/or leukopenia may occur anytime within 8 weeks after ...
-
DOSAGE AND ADMINISTRATIONParenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Mitomycin should be given ...
-
HOW SUPPLIEDMUTAMYCIN - ® (Mitomycin) for Injection USP - NDC 69448-001-05—Each amber vial contains 5 mg mitomycin, individually packed in single carton. NDC 69448-002-11—Each amber vial ...
-
ReferencesONS Clinical Practice Committee. Cancer Chemotherapy Guidelines and Recommendations for Practice Pittsburgh, PA: Oncology Nursing Society; 1999:32-41. Recommendations for the safe handling of ...
-
SPL UNCLASSIFIED SECTIONManufactured for : Accord BioPharma Inc. 1009 Slater Road, Suite 210-B, Durham, NC 27703. USA - Manufactured by : Intas Pharmaceuticals Limited, Ahmedabad – 380 009 ...
-
SPL UNCLASSIFIED SECTION10 0823 0 668295 - Issued - September, 2016
-
PRINCIPAL DISPLAY PANELCarton Label-MUTAMYCIN - ® (Mitomycin) for injection USP 40 mg/vial - NDC 69448- 003-38 - MUTAMYCIN ...
-
INGREDIENTS AND APPEARANCEProduct Information