Label: MOTEGRITY- prucalopride tablet, film coated
- NDC Code(s): 54092-546-01, 54092-547-01, 54092-547-02, 54092-547-03
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MOTEGRITY safely and effectively. See full prescribing information for MOTEGRITY. MOTEGRITY (prucalopride) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMOTEGRITY® is indicated for the treatment of chronic idiopathic constipation (CIC) in adults.
-
2 DOSAGE AND ADMINISTRATIONMOTEGRITY can be taken with or without food. The recommended dosage by patient population is shown in Table 1. Table 1: Recommended Dosage Regimen and Dosage Adjustments by ...
-
3 DOSAGE FORMS AND STRENGTHSMOTEGRITY Tablets: 1 mg prucalopride: White to off-white, round, biconvex film-coated tablet debossed with "PRU 1" on one side and no debossing on the other side. 2 mg prucalopride: Pink, round ...
-
4 CONTRAINDICATIONSMOTEGRITY is contraindicated in patients with: A history of hypersensitivity to MOTEGRITY. Reactions including dyspnea, rash, pruritus, urticaria, and facial edema have been observed [(see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Suicidal Ideation and Behavior - In clinical trials, suicides, suicide attempts, and suicidal ideation have been reported. Postmarketing cases of suicidal ideation and behavior as well as ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to MOTEGRITY during pregnancy. Healthcare providers are ...
-
10 OVERDOSAGEAn overdose may result in appearance of symptoms from an exaggeration of the known pharmacodynamic effects of prucalopride and includes headache, nausea, and diarrhea. Specific treatment is not ...
-
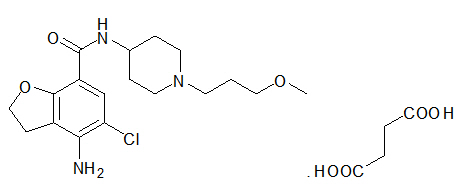
11 DESCRIPTIONMOTEGRITY (prucalopride) tablets for oral use contain prucalopride succinate, a dihydrobenzofurancarboxamide that is a serotonin type 4 (5-HT4) receptor agonist. The IUPAC name is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Prucalopride, a selective serotonin type 4 (5-HT4) receptor agonist, is a gastrointestinal (GI) prokinetic agent that stimulates colonic peristalsis (high-amplitude ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 2-year carcinogenicity study in mice, prucalopride was given by daily oral gavage at doses of 10, 20, and 80 ...
-
14 CLINICAL STUDIESThe efficacy of MOTEGRITY for the treatment of CIC was evaluated in six double-blind, placebo-controlled, randomized, multicenter clinical trials in 2484 adult patients (Studies 1 to 6; see Table ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMOTEGRITY tablets containing 1 mg prucalopride are white to off-white, round, biconvex film-coated tablets debossed with "PRU 1" on one side and no debossing on the other side. They are supplied ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information) Suicidal Ideation and Behavior: Inform patients, their caregivers, and family members that suicidal ideation ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Takeda Pharmaceuticals America, Inc. Lexington, MA 02421 - For more information go to www.motegrity.com or call 1-800-828-2088 - MOTEGRITY® and the MOTEGRITY Logo® are registered ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug AdministrationIssued: November 2020 - PATIENT INFORMATION - MOTEGRITY® (moe-teh'-gri-tee) (prucalopride) tablets, for ...
-
PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle LabelRx Only - NDC 54092-546-01 - motegrity® (prucalopride) tablets - 1 mg - Usual Dose: One tablet once daily. 30 Tablets
-
PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle LabelRx Only - NDC 54092-547-01 - motegrity® (prucalopride) tablets - 2 mg - Usual Dose: One tablet once daily. 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information