Label: MONOFERRIC- ferric derisomaltose injection, solution
- NDC Code(s): 73594-9301-2, 73594-9301-3, 73594-9305-1, 73594-9310-1
- Packager: Pharmacosmos Therapeutics Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MONOFERRIC safely and effectively. See Full Prescribing Information for MONOFERRIC. MONOFERRIC (ferric derisomaltose ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMonoferric is indicated for the treatment of iron deficiency anemia (IDA) in adult patients: who have intolerance to oral iron or have had unsatisfactory response to oral iron - who have ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - For patients weighing 50 kg or more: Administer 1,000 mg of Monoferric by intravenous infusion over at least 20 minutes as a single dose. Repeat dose if iron deficiency ...
-
3 DOSAGE FORMS AND STRENGTHSMonoferric is a sterile, dark brown, non-transparent aqueous solution available as: Injection: 1,000 mg iron/10 mL (100 mg/mL) single-dose vial - Injection: 500 mg iron/5 mL (100 mg/mL) single-dose ...
-
4 CONTRAINDICATIONSMonoferric is contraindicated in patients with a history of serious hypersensitivity to Monoferric or any of its components - (see - Warnings and Precautions (5.1) ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: Hypersensitivity Reactions - (see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on Monoferric use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or ...
-
10 OVERDOSAGEExcessive dosages of Monoferric may lead to accumulation of iron in storage sites potentially leading to hemosiderosis and hemochromatosis. Avoid use of Monoferric in patients with iron overload ...
-
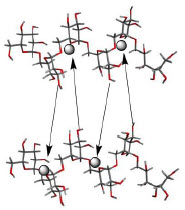
11 DESCRIPTIONMonoferric is an iron replacement product containing ferric derisomaltose for intravenous infusion. Ferric derisomaltose is an iron carbohydrate complex with a matrix structure composed of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ferric derisomaltose is a complex of iron (III) hydroxide and derisomaltose, an iron carbohydrate oligosaccharide that releases iron. Iron binds to transferrin for ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been conducted. Iron oligosaccharide, an earlier formulation of ferric derisomaltose, was not ...
-
14 CLINICAL STUDIESThe safety and efficacy of Monoferric for treatment of iron deficiency anemia (IDA) were evaluated in two randomized, open-label, actively-controlled clinical trials performed in a total of 3050 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Monoferric injection is a sterile, dark brown, non-transparent aqueous solution supplied in cartons as single-dose vials (10 mL, 5 mL or 1 mL) in the following ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Prior History of Allergies to Parenteral Iron Products - Question patients regarding any prior history of ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Pharmacosmos Therapeutics Inc., Morristown, NJ 07960 - Monoferric is manufactured under license from Pharmacosmos A/S, Denmark
-
PATIENT PACKAGE INSERT8This Patient Information has been approved by the U.S. Food and Drug Administration.Issued: 02/2020 - Patient Information - MONOFERRIC (mon-oh-fer-ik) (ferric derisomaltose) Injection ...
-
PRINCIPAL DISPLAY PANEL - 100 mg/mL Vial BoxPHARMACOSMOS - MonoFerric - ® (ferric derisomaltose) injection - 100 mg / mL - FOR INTRAVENOUS INFUSION AFTER DILUTION - Discard unused portion - Sterile ...
-
PRINCIPAL DISPLAY PANEL - 500 mg/5 mL Vial BoxMonoFerric - ® (ferric derisomaltose) injection - 500 mg / 5 mL - (100 mg/mL) FOR INTRAVENOUS INFUSION - AFTER DILUTION ...
-
PRINCIPAL DISPLAY PANEL - 1000 mg/10 mL Vial BoxMonoFerric - ® (ferric derisomaltose) injection - 1000 mg / 10 mL - (100 mg/mL) FOR INTRAVENOUS INFUSION - AFTER DILUTION ...
-
INGREDIENTS AND APPEARANCEProduct Information