Label: MITOSOL- mitomycin kit
- NDC Code(s): 49771-002-01, 49771-002-03
- Packager: Mobius Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MITOSOL® safely and effectively. See full prescribing information for MITOSOL®. MITOSOL® (mitomycin for solution) for ophthalmic ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Mitosol® is an antimetabolite indicated for use as an adjunct to ab externo glaucoma surgery.
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Administration Instructions - Mitosol® is intended for topical application to the surgical site of glaucoma filtration surgery. Mitosol® is a cytotoxic drug. It is not intended for ...
-
3 DOSAGE FORMS AND STRENGTHS Mitosol® is a sterile lyophilized mixture of mitomycin and mannitol, which, when reconstituted with Sterile Water for Injection, provides a solution for application in glaucoma filtration surgery ...
-
4 CONTRAINDICATIONS 4.1 Hypersensitivity - Mitosol® is contraindicated in patients that have demonstrated a hypersensitivity to mitomycin in the past.
-
5 WARNINGS AND PRECAUTIONS 5.1 Cell Death - Mitomycin is cytotoxic. Use of mitomycin in concentrations higher than 0.2 mg/mL or use for longer than 2 minutes may lead to unintended corneal and/or scleral damage including ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Cell Death [see Warnings and Precautions (5.1)] • Hypotony [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on findings in animals and mechanism of action [see Clinical Pharmacology (12.1)], Mitosol® can cause fetal harm when administered to a pregnant woman ...
-
11 DESCRIPTION Mitomycin is an antibiotic isolated from the broth of Streptomyces verticillus Yingtanensis which has been shown to have antimetabolic activity. Mitomycin is a blue-violet crystalline powder with ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Mitosol® inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Adequate long-term studies in animals to evaluate carcinogenic potential have not been conducted with Mitosol®. Intravenous ...
-
14 CLINICAL STUDIES In placebo-controlled studies reported in the medical literature, mitomycin reduced intraocular pressure (IOP) by 3 mmHg in patients with open-angle glaucoma when used as an adjunct to ab externo ...
-
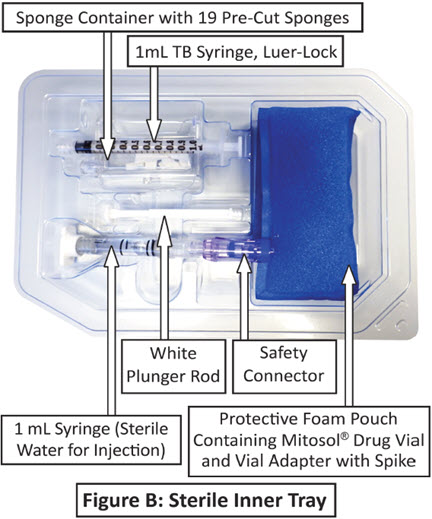
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - Mitosol® (mitomycin for solution) is available in a kit containing: One Vial containing 0.2 mg mitomycin - One 1 mL syringe (Sterile Water For ...
-
17 PATIENT COUNSELING INFORMATION • Instruct patients to discuss with their physician if they are pregnant or if they might become pregnant [see Use in Specific Populations (8.1)]. • Instruct patients to discuss with their ...
-
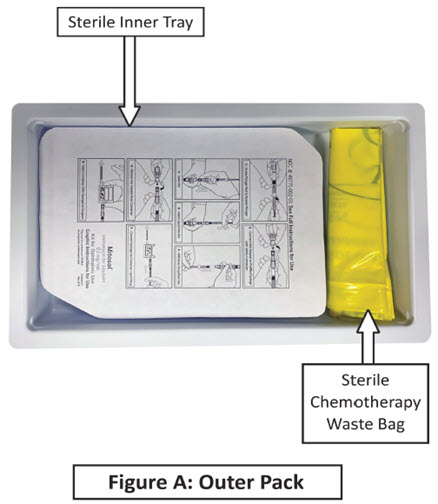
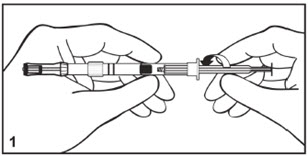
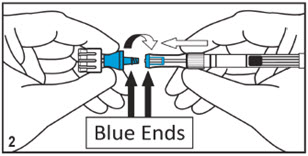
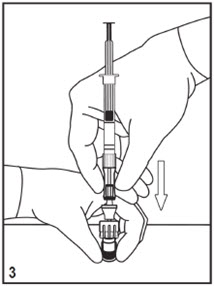
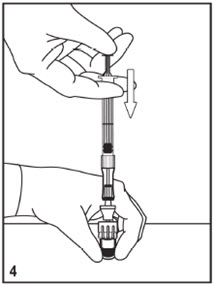
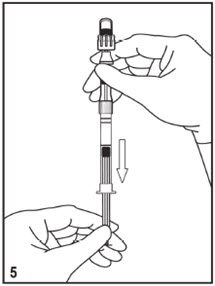
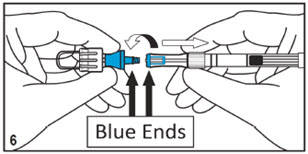
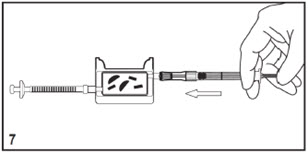
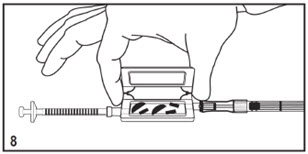
Mitosol® (mitomycin for solution) 0.2 mg/vial - Kit for Ophthalmic Use - Read INSTRUCTIONS FOR USE Before Proceeding - Instructions for Use - A. Outer Pack - (Figure ...
-
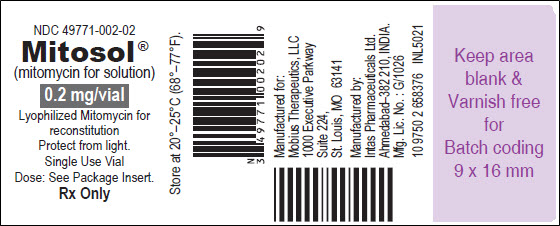
PRINCIPAL DISPLAY PANEL - VIAL LABEL Vial Label - NDC 49771-002-02 - Mitosol® (mitomycin for solution) 0.2 mg/vial - Lyophilized Mitomycin for - reconstitution - For ophthalmic use - Protect from light. Single Use Vial - Dose: See Package ...
-
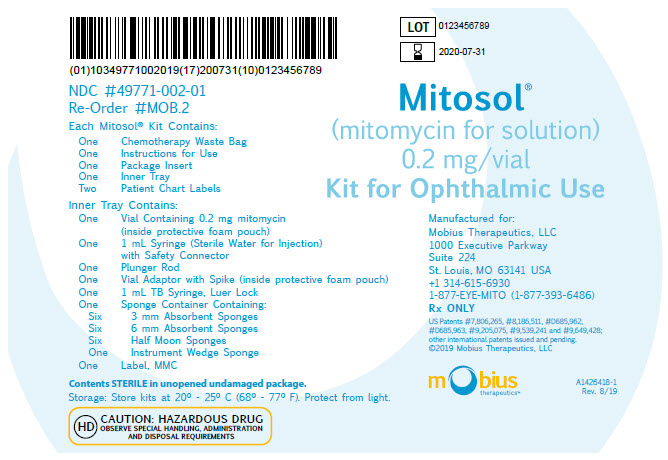
PRINCIPAL DISPLAY PANEL - OUTER KIT PACKAGE Outer Kit Package - Mitosol® (mitomycin for solution) 0.2 mg/vial - Kit for Ophthalmic Use - Manufactured for: Mobius Therapeutics, LLC - 1000 Executive Parkway - Suite 224 - St. Louis, MO 63141 USA - +1 ...
-
INGREDIENTS AND APPEARANCEProduct Information