Label: MIOSTAT- carbachol solution
- NDC Code(s): 0065-0023-15
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:MIOSTAT™ (carbachol intraocular solution, USP) 0.01% is a sterile balanced salt solution of carbachol for intraocular injection. The active ingredient is represented by the chemical ...
-
CLINICAL PHARMACOLOGY:Carbachol is a potent cholinergic (parasympathomimetic) agent which produces constriction of the iris and ciliary body resulting in reduction in intraocular pressure (IOP). The exact mechanism by ...
-
INDICATIONS AND USAGE:Intraocular use for obtaining miosis during surgery. In addition, MIOSTAT* (carbachol intraocular solution, USP) 0.01% reduces the intensity of IOP elevation in the first 24 hours after cataract ...
-
CONTRAINDICATIONS:Should not be used in those persons showing hypersensitivity to any of the components of this preparation.
-
WARNINGS:For single-dose intraocular use only. Discard unused portion. Intraocular carbachol 0.01% should be used with caution in patients with acute cardiac failure, bronchial asthma, peptic ulcer ...
-
PRECAUTIONS:Use only if the container is undamaged. Carcinogenesis: Studies in animals to evaluate the carcinogenic potential have not been conducted. Pregnancy: There are no adequate and ...
-
Pediatric Use:Safety and efficacy in pediatric patients have not been established.
-
Geriatric Use:No overall differences in safety or effectiveness have been observed between elderly and younger patients.
-
ADVERSE REACTIONS:Ocular: Corneal clouding, persistent bullous keratopathy, retinal detachment and postoperative iritis following cataract extraction have been reported. Systemic: Side effects such as flushing ...
-
DOSAGE AND ADMINISTRATION:Aseptically remove the sterile vial from the blister package by peeling the backing paper and dropping the vial onto a sterile tray. Withdraw the contents into a dry sterile syringe, and replace ...
-
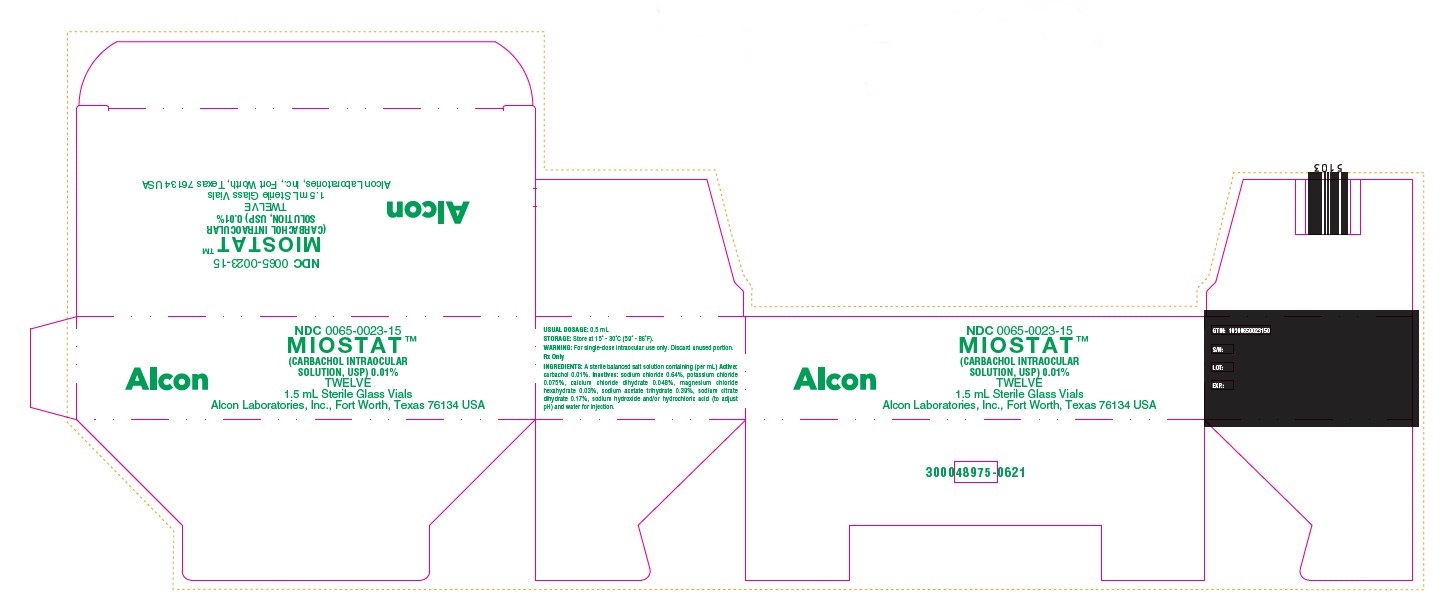
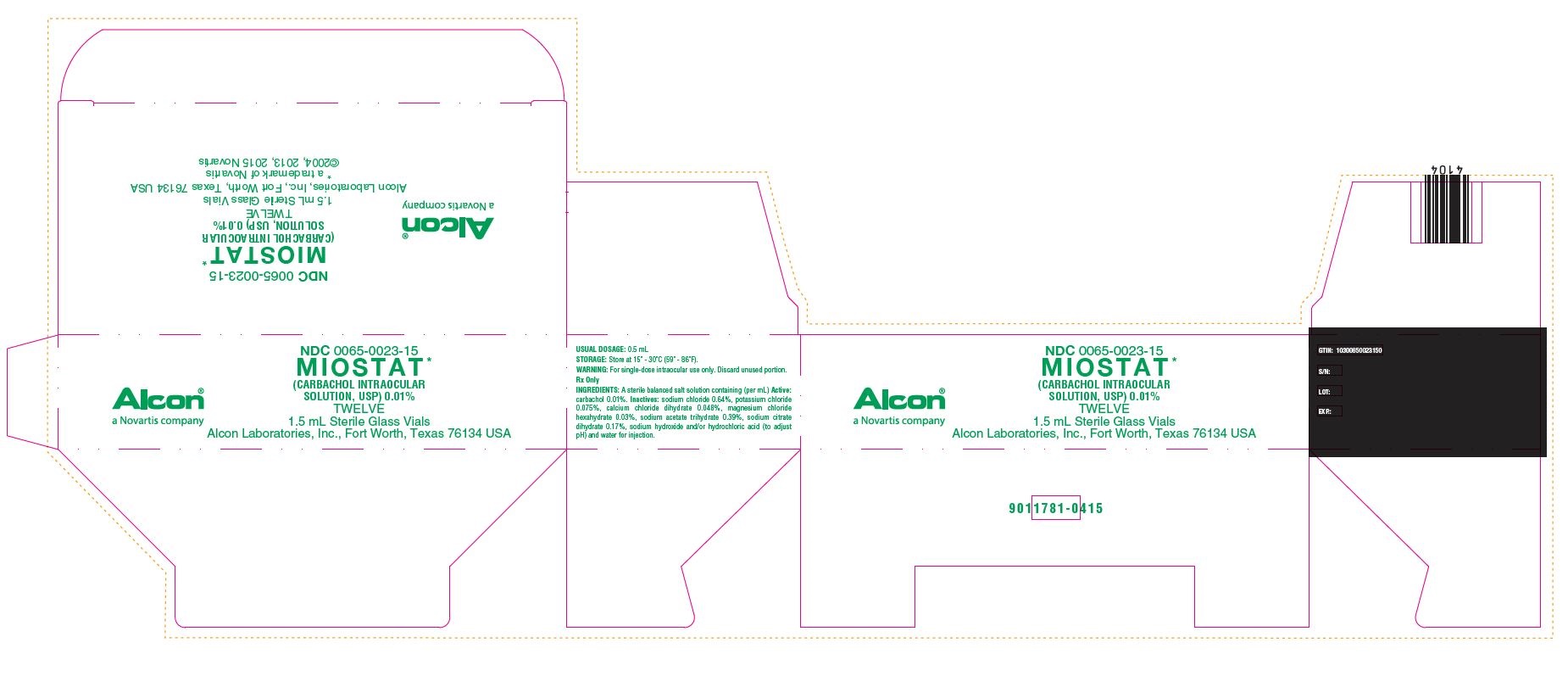
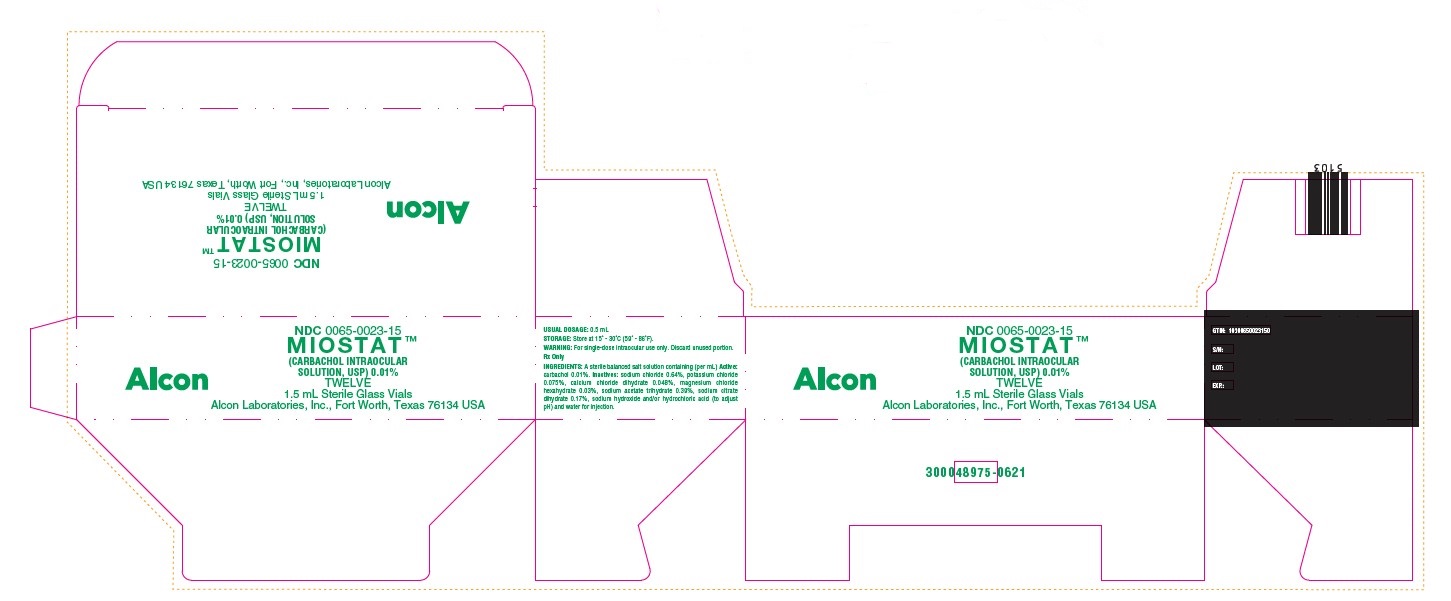
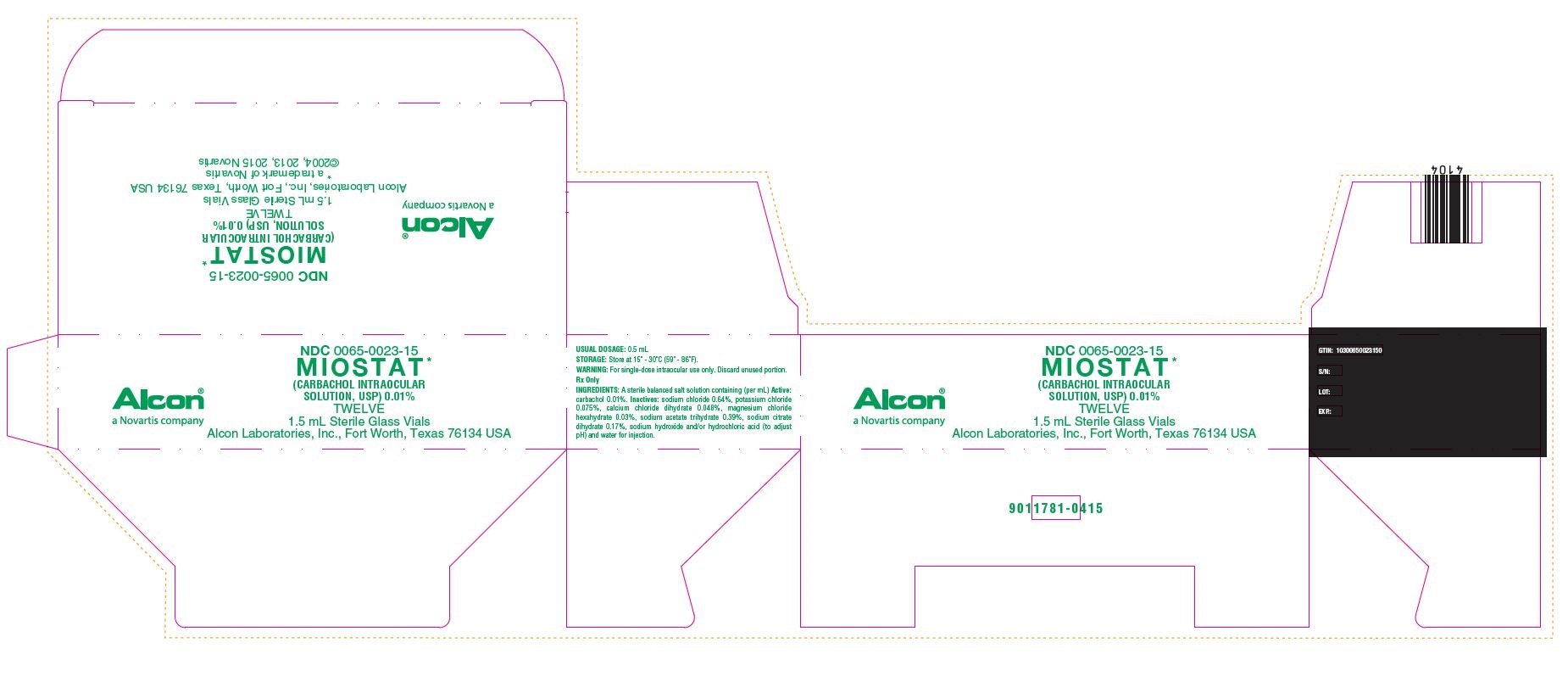
HOW SUPPLIED:In a 2.0 mL glass vial with a 1.5 mL fill, grey butyl stopper and aluminum seal packaged twelve to a carton…………………………………………………………………………..NDC 0065-0023-15 - STORAGE: Store at 15° - 30°C (59° ...
-
PRINCIPAL DISPLAY PANELNDC 0065-0023-15 - MIOSTAT™ (CARBACHOL INTRAOCULAR - SOLUTION, USP) 0.01% TWELVE - 1.5 mL Sterile Glass Vials - Alcon Laboratories, Inc., Fort Worth, Texas 76134 USA - Alcon - USUAL ...
-
INGREDIENTS AND APPEARANCEProduct Information