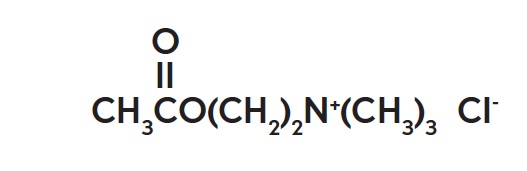Label: MIOCHOL E- acetylcholine chloride kit
- NDC Code(s): 24208-539-20
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Miochol™-E (acetylcholine chloride intraocular solution) is a parasympathomimetic preparation for intraocular use. It is packaged in a blister pack containing one vial and one diluent ampule. The ...
-
CLINICAL PHARMACOLOGY Acetylcholine is a naturally occurring neurohormone which mediates nerve impulse transmission at all cholinergic sites involving somatic and autonomic nerves. After release from the nerve ending ...
-
INDICATIONS AND USAGE To obtain miosis of the iris in seconds after delivery of the lens in cataract surgery, in penetrating keratoplasty, iridectomy, and other anterior segment surgery where rapid miosis may be ...
-
CONTRAINDICATIONS Miochol-E is contraindicated in persons with a known hypersensitivity to any component of this product.
-
WARNINGS DO NOT GAS STERILIZE. If blister or peelable backing is damaged or broken, sterility of the enclosed vial and ampule cannot be assured. Open under aseptic conditions only.
-
PRECAUTIONS General - If miosis is to be obtained quickly with Miochol-E, anatomical hindrances to miosis, such as anterior or posterior synechiae, must be released, prior to administration of Miochol-E ...
-
ADVERSE REACTIONS Infrequent cases of corneal edema, corneal clouding, and corneal decompensation have been reported with the use of intraocular acetylcholine. Adverse reactions have been reported rarely, which are ...
-
OVERDOSAGE Atropine sulfate (0.5 to 1 mg) should be given intramuscularly or intravenously and should be readily available to counteract possible overdosage. Epinephrine (0.1 to 1 mg subcutaneously) is also ...
-
DOSAGE AND ADMINISTRATION Miochol™-E (acetylcholine chloride intraocular solution) is instilled into the anterior chamber before or after securing one or more sutures. Instillation should be gentle and parallel to the ...
-
DIRECTIONS FOR PREPARING MIOCHOL™-E: STERILE UNLESS PACKAGE OPEN OR BROKEN - 1. Inspect the unopened blister, vial, and ampule to ensure that they are all intact. Peel open the blister under a sterile field. Maintain sterility of ...
-
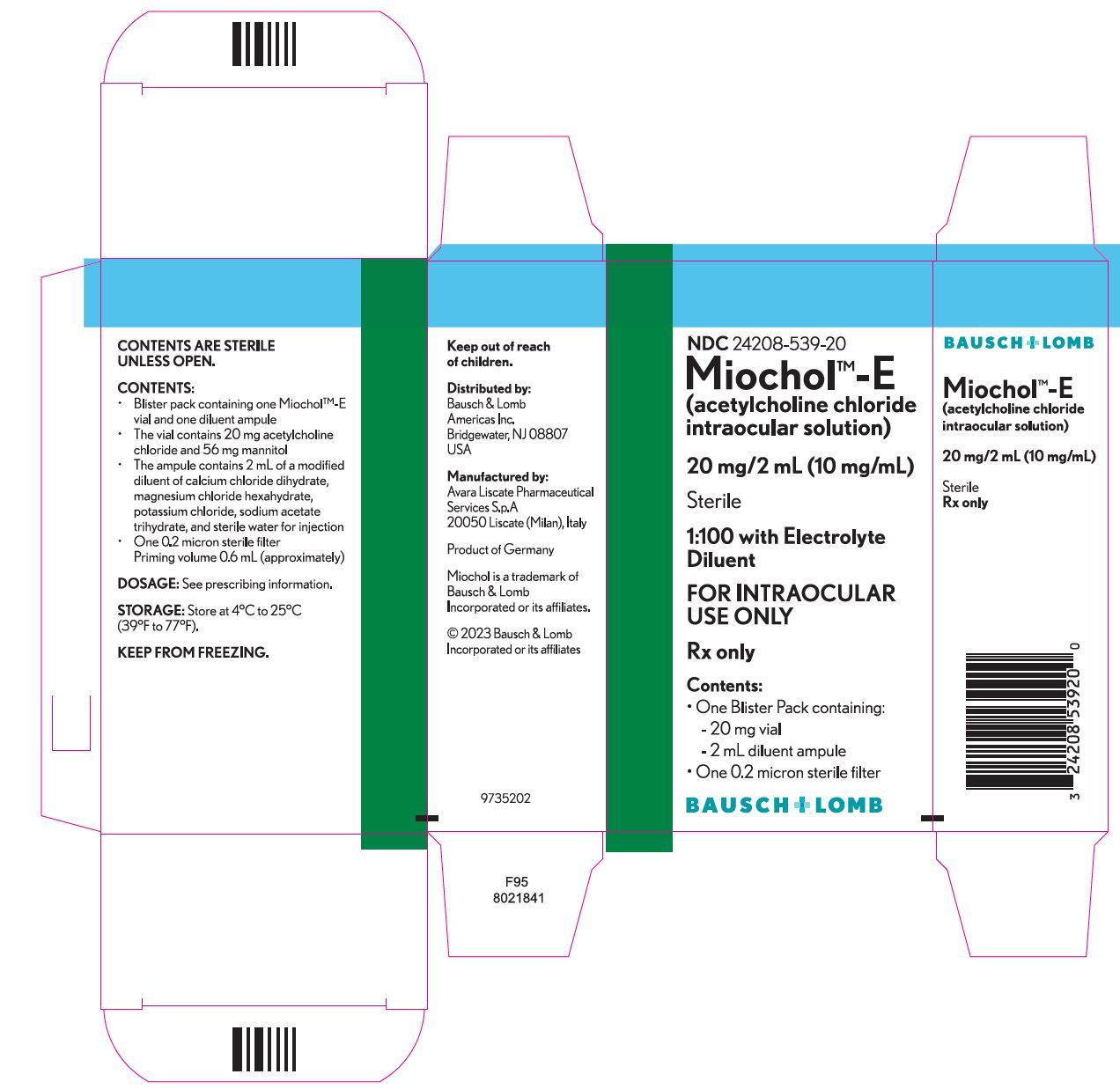
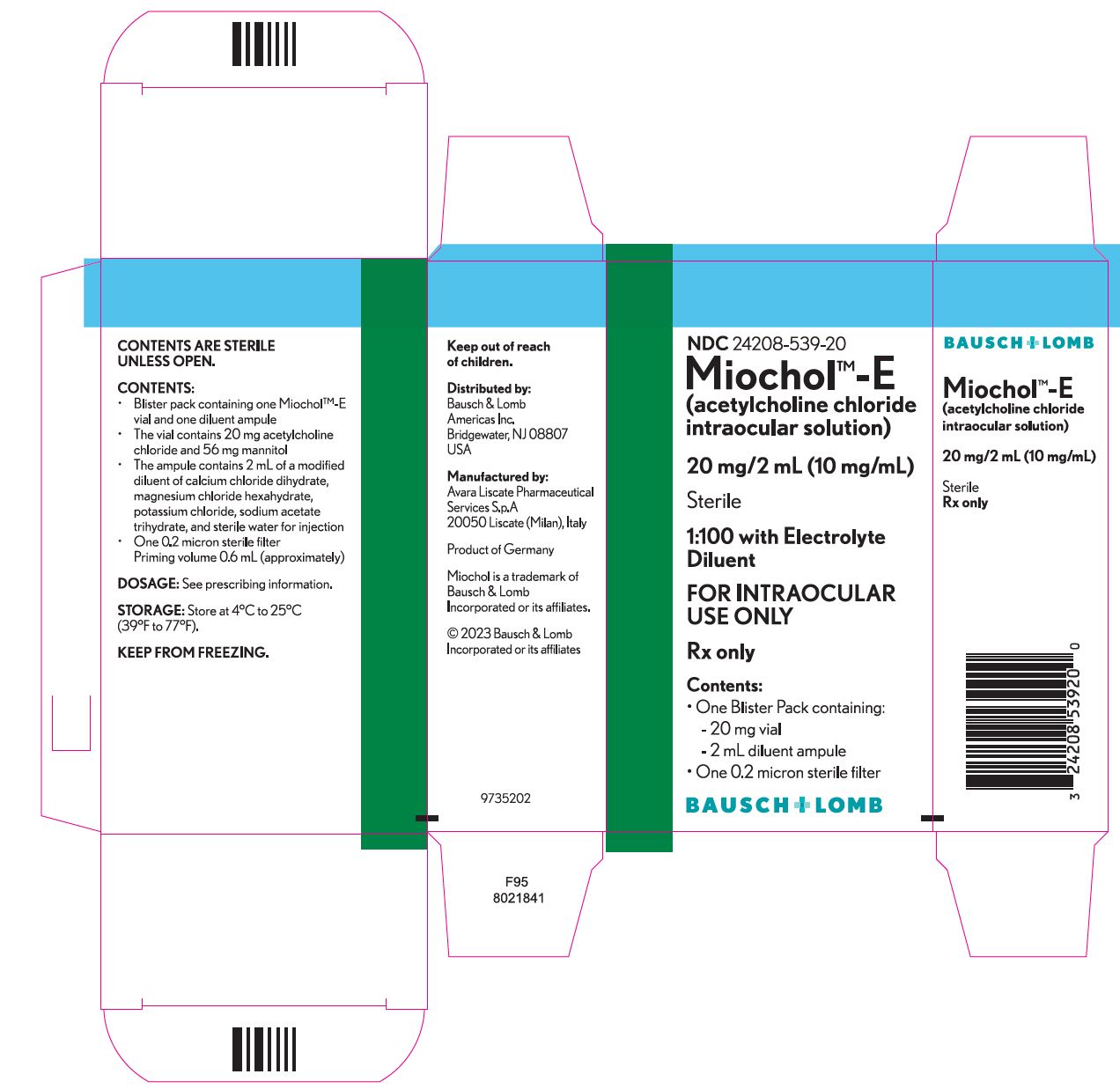
HOW SUPPLIED Miochol™-E - (acetylcholine chloride intraocular solution)............................NDC 24208-539-20 - One blister pack containing the following components: • Vial of 20 mg acetylcholine chloride ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 24208-539-20 - Miochol TM -E - (acetylcholine chloride intraocular solution) 20 mg/2 mL (10 mg/mL) Sterile - 1:100 with Electrolyte - Diluent - FOR INTRAOCULAR - USE ONLY - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information