Label: MINOLIRA EXTENDED RELEASE- minocycline hydrochloride tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 71403-101-05, 71403-101-30, 71403-102-05, 71403-102-30 - Packager: EPI Health, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MINOLIRA™ safely and effectively. See full prescribing information for MINOLIRA. MINOLIRA™ (minocycline hydrochloride ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMINOLIRA is indicated to treat the inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 12 years of age and older. Limitations of Use - This formulation of ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosage of MINOLIRA is approximately 1 mg/kg once daily for 12 weeks. Higher doses have not shown to be of additional benefit in the treatment of inflammatory lesions of acne, and ...
-
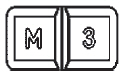
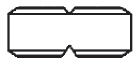
3 DOSAGE FORMS AND STRENGTHSMINOLIRA extended-release tablets are white to off-white, functionally scored, rectangular tablets with brown or gold color speckles and a single score line on both surfaces. MINOLIRA are ...
-
4 CONTRAINDICATIONSMINOLIRA is contraindicated in patients who have shown hypersensitivity to any of the tetracyclines [see Serious Skin/Hypersensitivity Reactions (5.11)].
-
5 WARNINGS AND PRECAUTIONS5.1 Teratogenic Effects - Avoid MINOLIRA use during pregnancy. MINOLIRA, like other tetracycline-class drugs, can cause fetal harm when administered to a pregnant woman. MINOLIRA, like other ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Anticoagulants - Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - MINOLIRA, like tetracycline class drugs, may cause permanent discoloration of teeth and reversible inhibition of bone growth when administered during pregnancy ...
-
10 OVERDOSAGEIn case of over dosage, discontinue medication, treat symptomatically and institute supportive measures. Minocycline is not removed in significant quantities by hemodialysis or peritoneal ...
-
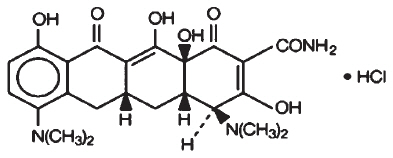
11 DESCRIPTIONMinocycline hydrochloride, a semi synthetic derivative of tetracycline, is [4S (4α,4aα,5aα,12aα)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of MINOLIRA for the treatment of acne is unknown. 12.2 Pharmacodynamics - The pharmacodynamics of MINOLIRA for the treatment of acne are ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a carcinogenicity study in which minocycline hydrochloride was orally administered to male and female rats once daily for up to 104 ...
-
14 CLINICAL STUDIESThe safety and efficacy of minocycline hydrochloride extended-release tablets in the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris was assessed in two 12-week ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - MINOLIRA is supplied as functionally scored extended-release tablets containing minocycline hydrochloride equivalent to 105 mg and 135 mg of minocycline. The 105 mg ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instruction for Use). Advise patients of the following: Teratogenic effects - Advise patients to avoid use ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Dr. Reddy's Laboratories Limited - FTO-SEZ-Process-Unit-01 - Survey No: 57 to 59, 60, 52 & 72 - Sector No: 9 to14 & 17 to 20, Devunipalavalasa Village - Ranasthalam Mandal ...
-
PATIENT INFORMATION MINOLIRA (min-oh-li'-rah) (minocycline hydrochloride) extended-release tabletsWHAT IS MINOLIRA? MINOLIRA is prescription medicine used to treat only pimples and red bumps (non-nodular inflammatory lesions) that happen with moderate to severe acne vulgaris in people 12 ...
-
INSTRUCTIONS FOR USE MINOLIRA (min-oh-li'-rah) (minocycline hydrochloride) extended-release tabletsRead this Instructions for Use before you start using MINOLIRA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare ...
-
PRINCIPAL DISPLAY PANEL - 135 mg Tablet Bottle LabelNDC 71403-102-30 - Rx Only - minolira™ (minocycline hydrochloride) extended-release tablets - 135 mg* *Each tablet contains 135 mg of minocycline - (33.75 mg as immediate release beads and - 101.25 mg ...
-
PRINCIPAL DISPLAY PANEL - 105 mg Tablet Bottle LabelNDC 71403-101-30 - Rx Only - minolira™ (minocycline hydrochloride) extended-release tablets - 105 mg* *Each tablet contains 105 mg of minocycline - (26.25 mg as immediate release beads and 78.75 - mg ...
-
INGREDIENTS AND APPEARANCEProduct Information