Label: METRONIDAZOLE500 MG 500 MG- metronidazole tablet, film coated
- NDC Code(s): 70882-131-14
- Packager: Cambridge Therapeutics Technologies, LLC
- This is a repackaged label.
- Source NDC Code(s): 23155-569
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 8, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of metronidazole and other antibacterial drugs, metronidazole should be used only to treat or prevent infections ...
-
BOXED WARNING
(What is this?)
WARNING
Close
Metronidazole has been shown to be carcinogenic in mice and rats (see PRECAUTIONS). Unnecessary use of the drug should be avoided. Its use should be reserved for the conditions described in the INDICATIONS AND USAGE section below. -
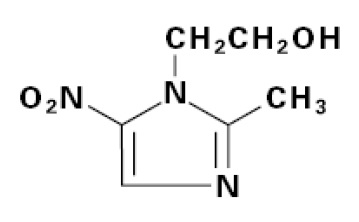
DESCRIPTION Metronidazole tablets USP, 250 mg or 500 mg is an oral formulation of the synthetic nitroimidazole antimicrobial, 2-methyl-5-nitro-1H-imidazole-1-ethanol, which has the following structural ...
-
CLINICAL PHARMACOLOGYAbsorption - Disposition of metronidazole in the body is similar for both oral and intravenous dosage forms. Following oral administration, metronidazole is well absorbed, with peak plasma ...
-
INDICATIONS AND USAGESymptomatic Trichomoniasis. Metronidazole tablets are indicated for the treatment of T. vaginalis infection in females and males when the presence of the trichomonad has been confirmed by ...
-
CONTRAINDICATIONSHypersensitivity - Metronidazole tablets are contraindicated in patients with a prior history of hypersensitivity to metronidazole or other nitroimidazole derivatives. In patients with ...
-
WARNINGSCentral and Peripheral Nervous System Effects - Encephalopathy and peripheral neuropathy: Cases of encephalopathy and peripheral neuropathy (including optic neuropathy) have been reported with ...
-
PRECAUTIONSGeneral - Hepatic Impairment - Patients with hepatic impairment metabolize metronidazole slowly, with resultant accumulation of metronidazole in the plasma. For patients with severe hepatic ...
-
ADVERSE REACTIONSThe following reactions have been reported during treatment with metronidazole: Central Nervous System: The most serious adverse reactions reported in patients treated with metronidazole have ...
-
OVERDOSAGESingle oral doses of metronidazole, up to 15 g, have been reported in suicide attempts and accidental overdoses. Symptoms reported include nausea, vomiting, and ataxia. Oral metronidazole has been ...
-
DOSAGE AND ADMINISTRATIONTrichomoniasis: In the Female: One-day treatment − two grams of metronidazole tablets, given either as a single dose or in two divided doses of one gram each, given in the same ...
-
HOW SUPPLIEDMetronidazole tablets USP, 500 mg are white, capsule shaped, film coated tablets, debossed with “H569” on one side and plain on the other side. They are supplied as follows: NDC 70882-131-14 ...
-
REFERENCES1. Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard -Eighth Edition. CLSI document M11-A8. Clinical and ...
-
Metronidazole Tablets, USP 500 mg

-
INGREDIENTS AND APPEARANCEProduct Information