Label: METOPIRONE- metyrapone capsule, gelatin coated
- NDC Code(s): 76336-455-18
- Packager: HRA Pharma Rare Diseases
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METOPIRONE safely and effectively. See full prescribing information for METOPIRONE. METOPIRONE - ®(metyrapone capsules), for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMetopirone is indicated, in combination with other diagnostic tests, for the diagnosis of adrenal insufficiency in adult and pediatric patients.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Information Before Conducting Metopirone Testing - Stop drugs affecting pituitary or adrenocortical function before administration of Metopirone in accordance with half-life of the ...
-
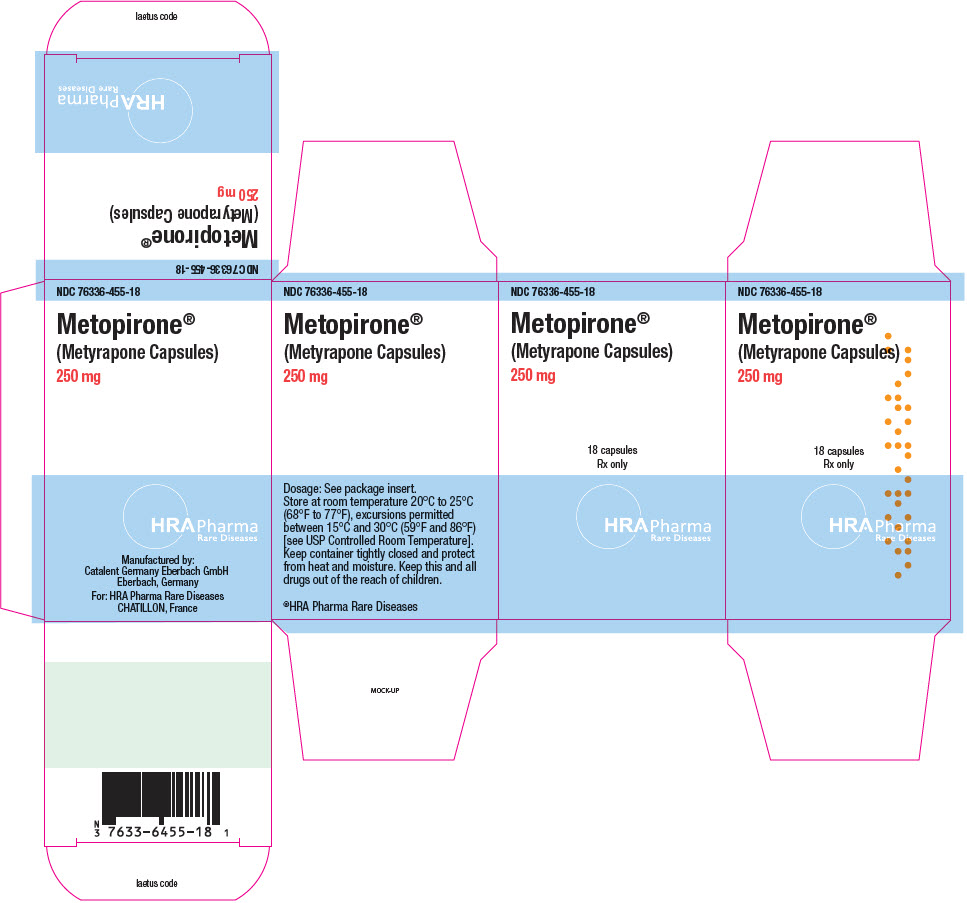
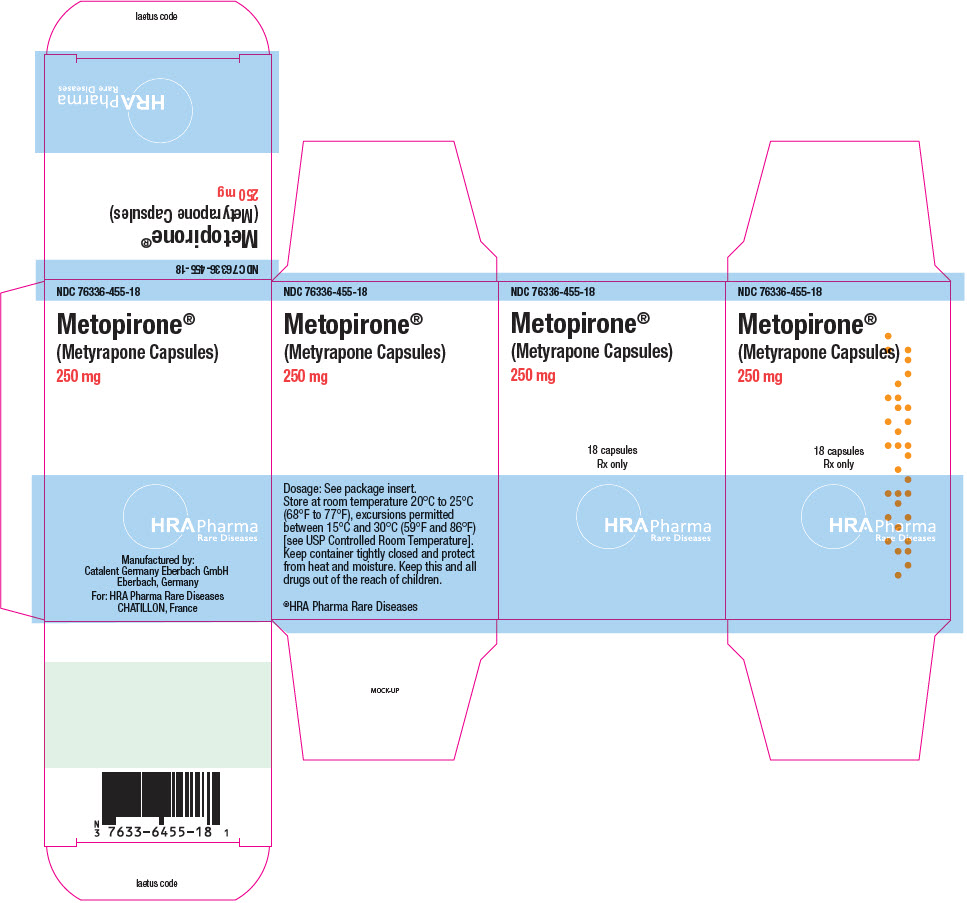
3 DOSAGE FORMS AND STRENGTHSCapsules: Metopirone 250 mg soft gelatin, white to yellowish‑white, oblong, opaque, imprinted with "HRA" on one side in red ink.
-
4 CONTRAINDICATIONSMetopirone is contraindicated in patients with adrenal cortical insufficiency or hypersensitivity to Metopirone or to any of its excipients.
-
5 WARNINGS AND PRECAUTIONS5.1 Adrenal Insufficiency - Metopirone may induce acute adrenal insufficiency in patients with reduced adrenal secretory capacity, as well as in patients with global pituitary insufficiency. The ...
-
6 ADVERSE REACTIONSThe following adverse reactions associated with the use of Metopirone were identified in clinical trials or postmarketing reports. Because these reactions were reported voluntarily from a ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on Metopirone - Anticonvulsants, psychotropic drugs, hormone preparations, corticosteroids, antithyroid agents and cyproheptadine may affect the results of the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published case series and reports on Metopirone use in pregnant females are insufficient to identify a drug-associated risk of major birth ...
-
10 OVERDOSAGEDeath occurred in a child after two doses of Metopirone 2 g. Signs and Symptoms of Overdosage - The clinical picture of overdosage with Metopirone is characterized by gastrointestinal symptoms ...
-
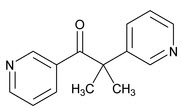
11 DESCRIPTIONMetopirone (metyrapone capsules) is an adrenal steroid synthesis inhibitor, available as 250‑mg capsules for oral administration. Its chemical name is 2‑methyl‑1, 2‑di‑3‑pyridyl‑1‑propanone, and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The pharmacological effect of Metopirone is to reduce cortisol and corticosterone production by inhibiting the 11-beta-hydroxylation reaction in the adrenal cortex ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long‑term animal carcinogenicity studies have not been conducted with Metopirone. Mutagenesis - Metyrapone was ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Metopirone (metyrapone capsules) are supplied as 250 mg soft gelatin, white to yellowish‑white, oblong, opaque capsules, imprinted with "HRA" on one side in red ink. Bottle of 18 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patient to read the FDA-approved labeling (Patient Information). Advise patient that Metopirone may induce acute adrenal insufficiency (nausea, vomiting, abdominal pain, hypotension ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Catalent Germany Eberbach GmbH - Eberbach, Germany - For: HRA Pharma Rare Diseases - CHATILLON, France - © 2022 HRA Pharma Rare Diseases | Metopirone is a ...
-
PRINCIPAL DISPLAY PANEL - 250 mg Bottle CartonNDC 76336-455-18 - Metopirone - ® (Metyrapone Capsules) 250 mg - 18 capsules - Rx only - HRA Pharma - Rare Diseases
-
INGREDIENTS AND APPEARANCEProduct Information