Label: METHITEST- methyltestosterone tablet
- NDC Code(s): 0115-7037-01
- Packager: Amneal Pharmaceuticals of New York LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
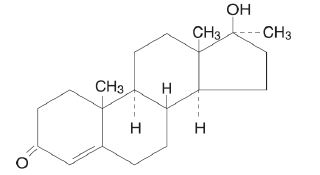
DESCRIPTIONMETHITEST™ (methyltestosterone) tablets, USP contain methyltestosterone USP, a synthetic androgen. Androgens are steroids that develop and maintain primary and secondary male sex characteristics ...
-
CLINICAL PHARMACOLOGYEndogenous androgens are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and ...
-
INDICATIONS AND USAGE1. Males - Androgens are indicated for replacement therapy in conditions associated with a deficiency or absence of endogenous testosterone: Primary hypogonadism (congenita or acquired) ...
-
CONTRAINDICATIONSMETHITEST™ (methyltestosterone) tablets are contraindicated in men with carcinomas of the breast or with known or suspected carcinomas of the prostate, and in women who are or may become pregnant ...
-
WARNINGSIn patients with breast cancer, androgen therapy may cause hypercalcemia by stimulating osteolysis. In this case, the drug should be discontinued. Prolonged use of high doses of androgens has been ...
-
PRECAUTIONSGeneral - Woman should be observed for signs of virilization (deepening of the voice, hirsutism, acne, clitoromegaly, and menstrual irregularities). Discontinuation of drug therapy at the time of ...
-
ADVERSE REACTIONSEndocrine and Urogenital - Female: The most common side effects of androgen therapy are amenorrhea and other menstrual irregularities, inhibition of gonadotropin secretion, and virilization ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance - METHITEST™ (methyltestosterone) tablets contain testosterone, a Schedule III controlled substance in the Controlled Substances Act. Abuse - Drug abuse is intentional ...
-
OVERDOSAGEOverdose of medication may be reflected in the occurrence of the signs and symptoms associated with testosterone-anabolic drugs. Nausea and appearance of the early manifestations of edema should ...
-
DOSAGE AND ADMINISTRATIONPrior to initiating METHITEST™ (methyltestosterone) tablets, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least ...
-
HOW SUPPLIEDMETHITEST™ (methyltestosterone) Tablets USP, 10 mg: Each compressed, single-scored, round, white tablet contains 10 mg of methyltestosterone USP, for oral use. Each tablet is debossed with “7037 ...
-
PRINCIPAL DISPLAY PANEL - 10 mg Bottle Label

-
INGREDIENTS AND APPEARANCEProduct Information