Label: MENOPUR- menotropins kit
- NDC Code(s): 55566-7501-1, 55566-7501-2, 55566-7502-0
- Packager: Ferring Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MENOPUR ® safely and effectively. See full prescribing information for MENOPUR. MENOPUR ® (menotropins for injection ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDevelopment of Multiple Follicles and Pregnancy in Ovulatory Women as Part of an Assisted Reproductive Technology (ART) Cycle - Prior to initiation of treatment with MENOPUR® (menotropins for ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container ...
-
3 DOSAGE FORMS AND STRENGTHSLyophilized powder for Injection containing 75 International Units FSH and 75 International Units of LH activity, supplied as lyophilized powder or pellet in sterile vials with diluent vials and ...
-
4 CONTRAINDICATIONSMENOPUR is contraindicated in women who exhibit: Prior hypersensitivity to MENOPUR or menotropins products or one of their excipients - High levels of FSH indicating primary ovarian failure [see ...
-
5 WARNINGS AND PRECAUTIONSMENOPUR should only be used by physicians who are experienced in infertility treatment. MENOPUR contains gonadotropic substances capable of causing in women, Ovarian Hyperstimulation Syndrome ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed elsewhere in the labeling: Abnormal Ovarian Enlargement [see Warnings and Precautions (5.1)] Ovarian Hyperstimulation Syndrome [see Warnings ...
-
7 DRUG INTERACTIONSNo drug/drug interaction studies in humans have been conducted for MENOPUR.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic effects - Pregnancy Category X [see Contraindications (4)]. 8.3 Nursing Mothers - It is not known whether this drug is excreted in human milk. Because many ...
-
10 OVERDOSAGEAside from possible OHSS [see Warnings and Precautions (5.2)] and multiple gestations [see Warnings and Precautions (5.5)], there is no additional information on the consequences of acute ...
-
11 DESCRIPTIONMENOPUR is a preparation of gonadotropins (FSH and LH activity), extracted from the urine of postmenopausal women, which has undergone additional steps for purification. MENOPUR is a sterile ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - MENOPUR, administered for 7 to 20 days, produces ovarian follicular growth and maturation in women who do not have primary ovarian failure. Treatment with MENOPUR in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term toxicity studies in animals have not been performed to evaluate the carcinogenic potential of menotropins.
-
14 CLINICAL STUDIESThe efficacy of MENOPUR was established in one randomized, open-label, multicenter, multinational (in Europe and Israel), comparative clinical trial of women undergoing in vitro fertilization ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - MENOPUR (menotropins for injection) is supplied in sterile vials as a lyophilized, white to off-white powder or pellet. Each vial of MENOPUR is accompanied by a vial of ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information and Instructions for Use). 17.1 Dosing and Use - Instruct women on the correct usage and dosing of MENOPUR [see Dosage and Administration ...
-
SPL UNCLASSIFIED SECTIONMANUFACTURED FOR: FERRING PHARMACEUTICALS INC. PARSIPPANY, NJ 07054 - 8109000033 - Rev: 05/2018
-
Patient InformationMENOPUR® (Men-oh-pyoor) (menotropins for injection) for subcutaneous use - Read this Patient Information before you start using MENOPUR® (menotropins for injection) and each time you get a refill ...
-
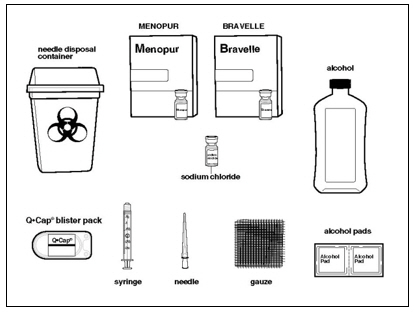
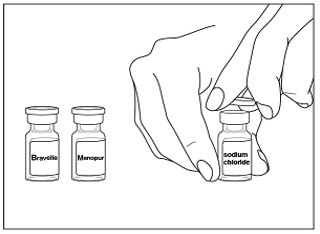
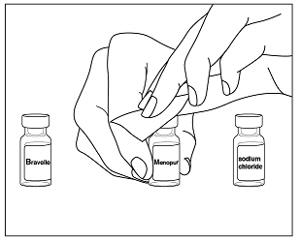
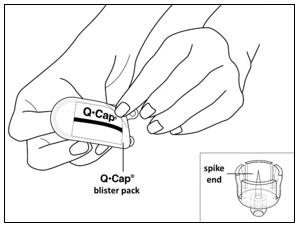
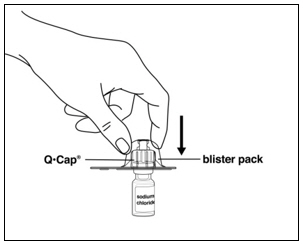
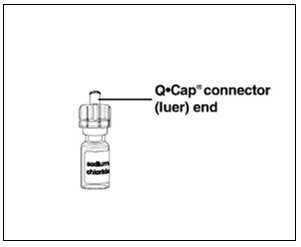
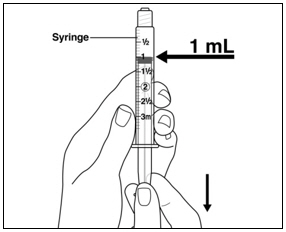
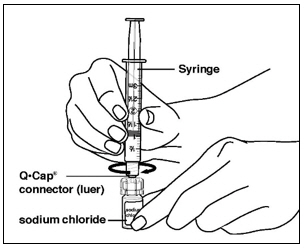
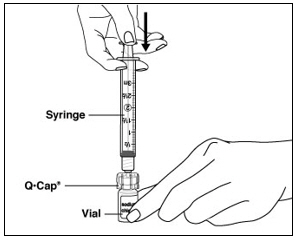
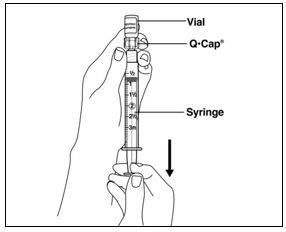
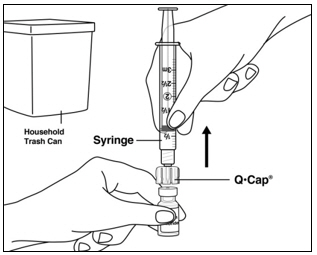
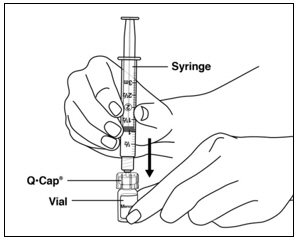
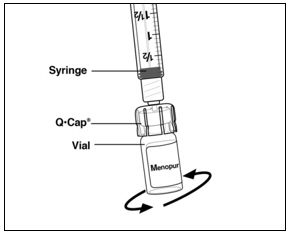
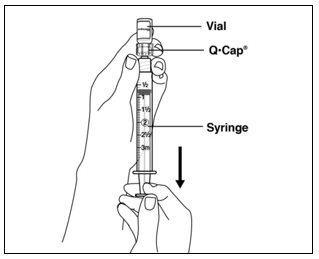
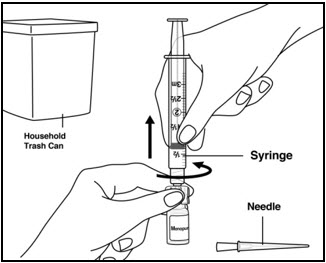
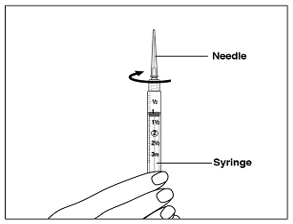
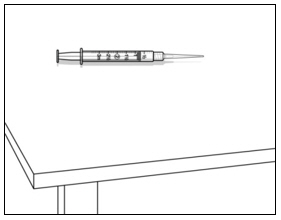
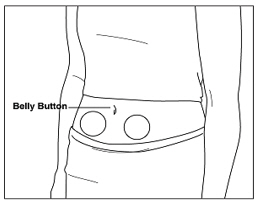
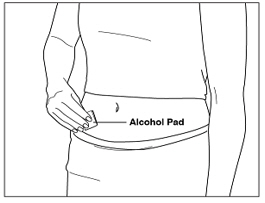
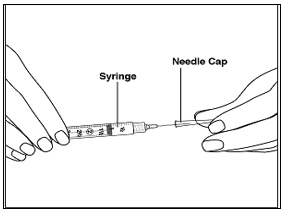
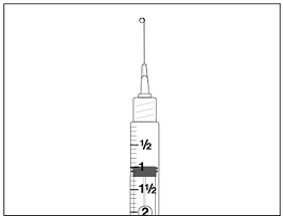
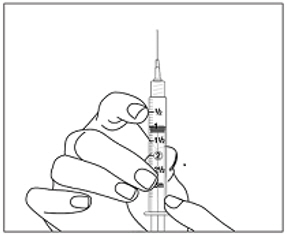
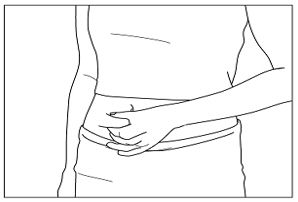
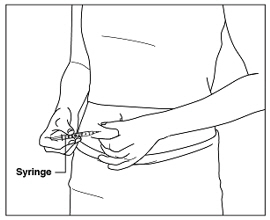
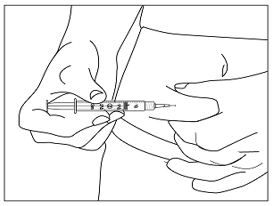
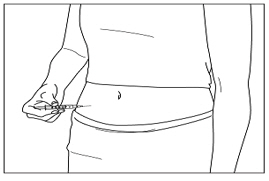
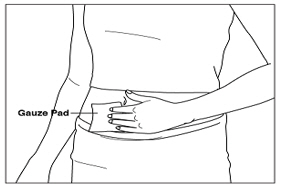
Instructions for UseMENOPUR® (Men-oh-pyoor) (menotropins for injection) for subcutaneous use - Your healthcare provider should show you how to mix and inject MENOPUR® (menotropins for injection) or MENOPUR mixed ...
-
SPL UNCLASSIFIED SECTIONMANUFACTURED FOR: FERRING PHARMACEUTICALS INC. PARSIPPANY, NJ 07054 - 8109000033 - Rev: 05/2018
-
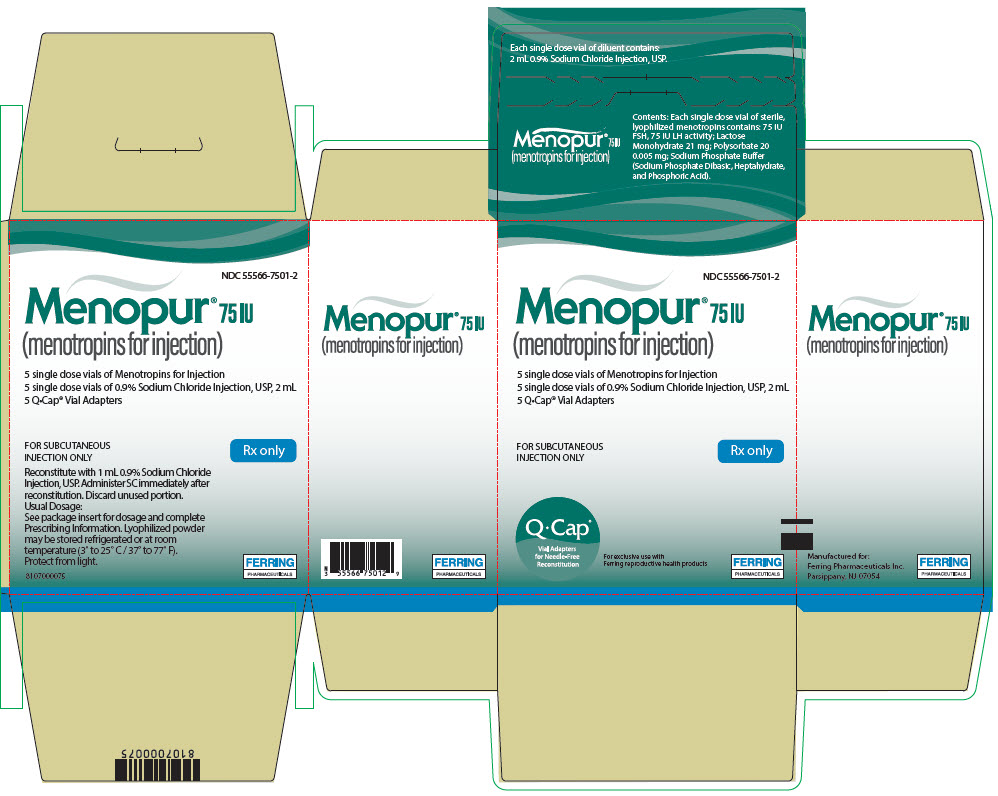
PRINCIPAL DISPLAY PANEL - Kit CartonNDC 55566-7501-2 - Menopur® 75 IU - (menotropins for injection) 5 single dose vials of Menotropins for Injection - 5 single dose vials of 0.9% Sodium Chloride Injection, USP, 2 mL - 5 Q•Cap® Vial ...
-
INGREDIENTS AND APPEARANCEProduct Information