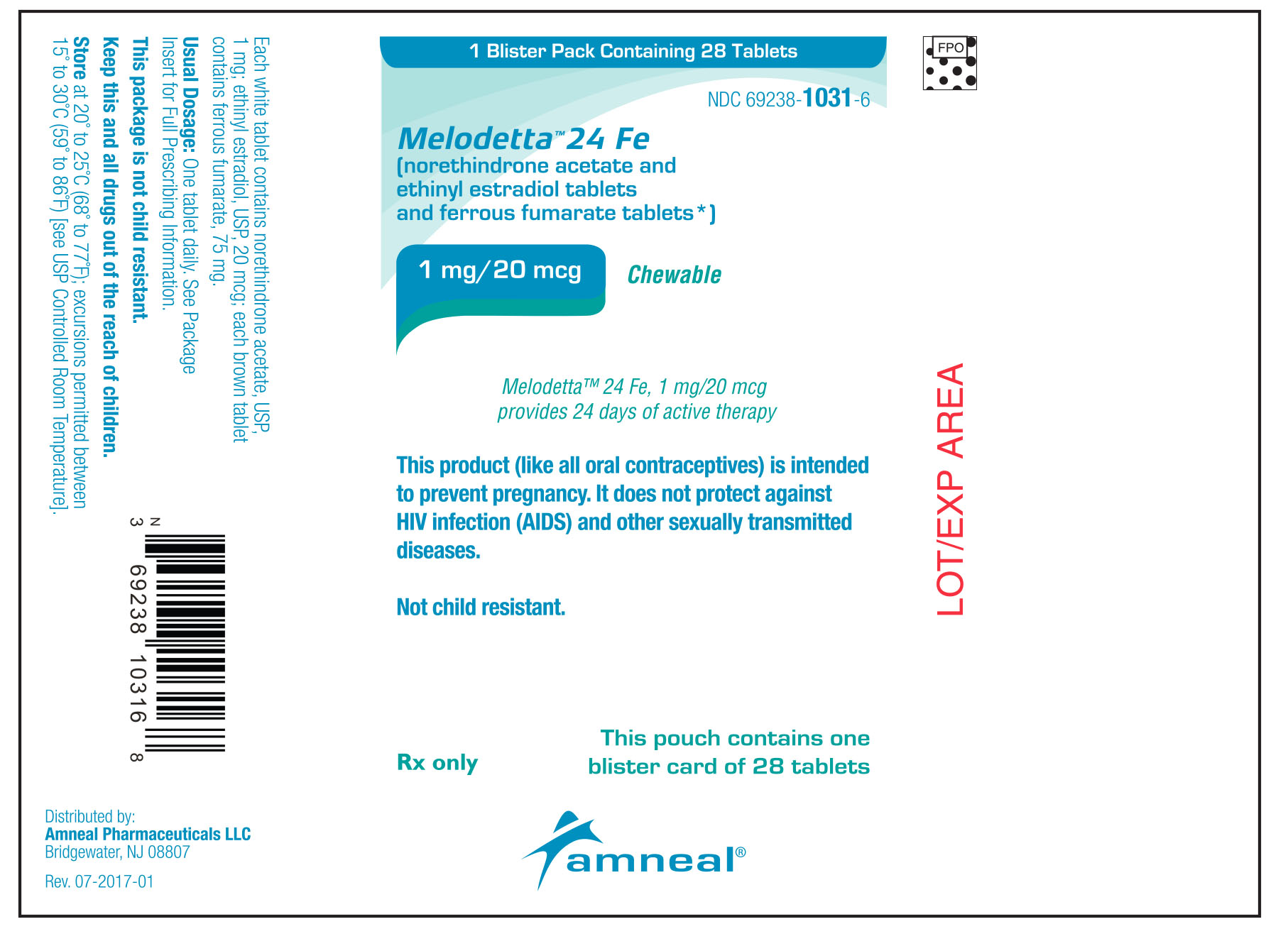
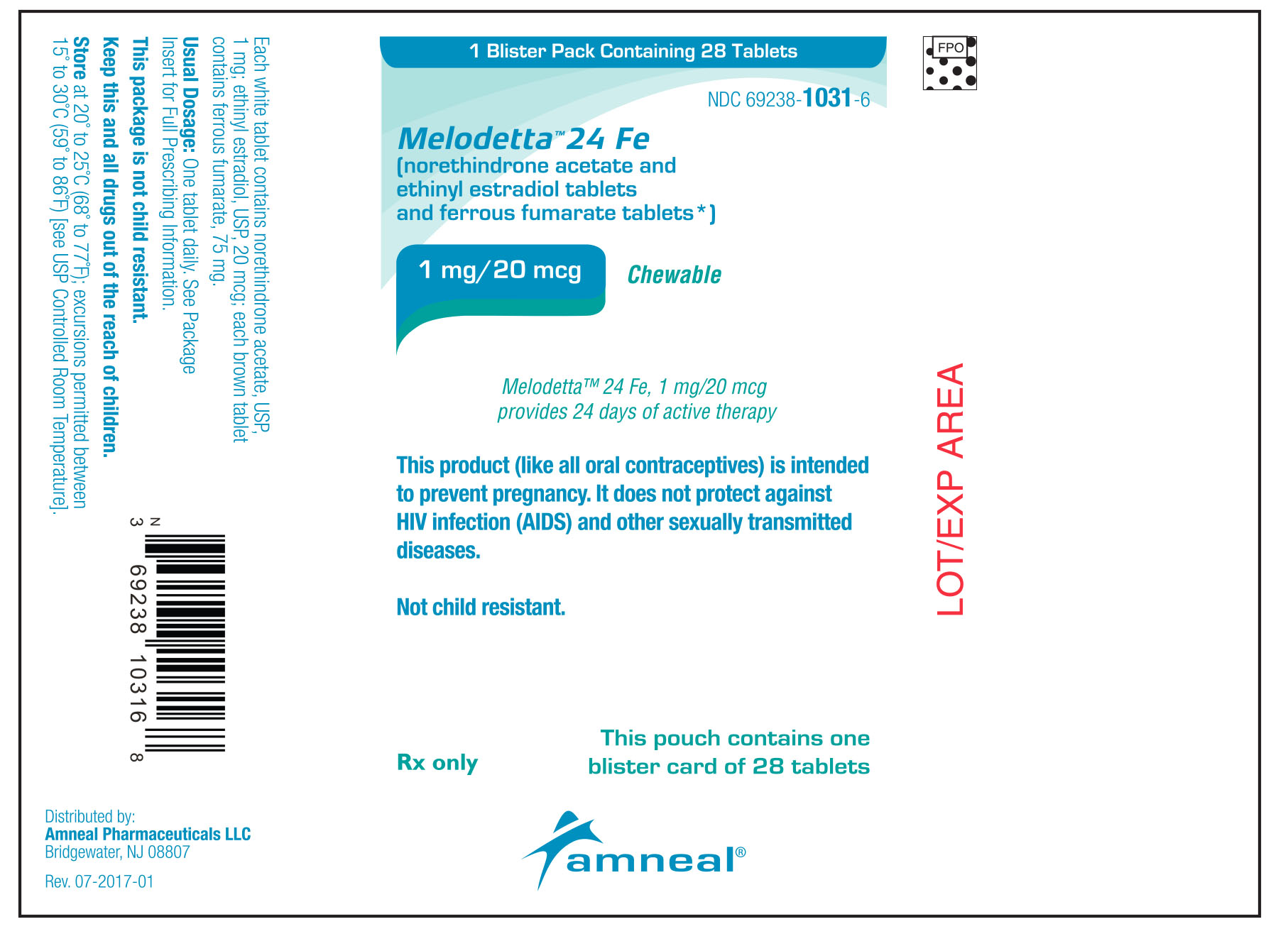
Guide for Using Melodetta™ 24 Fe (meh loe deh' tah)
(norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets)
WARNING TO WOMEN WHO SMOKE
-
Do not use ...
Guide for Using Melodetta™ 24 Fe (meh loe deh' tah)
(norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets)
Birth control pills help to lower the chances of becoming pregnant when taken as directed. They do not protect against HIV infection (AIDS) and other sexually transmitted infections.
What is Melodetta 24 Fe?
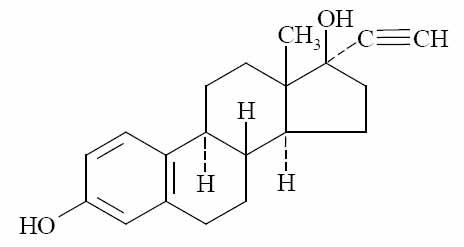
Melodetta 24 Fe is a birth control pill. It contains two female hormones, an estrogen called ethinyl estradiol, and a progestin called norethindrone acetate.
How well does Melodetta 24 Fe work?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The better you follow the directions, the less chance you have of getting pregnant.
Based on the results of one clinical study of a 24-day regimen of norethindrone acetate 1 mg/ethinyl estradiol 0.020 mg tablets lasting six months, about 1 to 4 out of 100 women may get pregnant during the first year they use Melodetta 24 Fe.
Women with a BMI above 35 kg/m2 were not studied in the clinical trial, so it is not known how well Melodetta 24 Fe protects against pregnancy in such women. If you are overweight, discuss with your healthcare provider whether Melodetta 24 Fe is the best choice for you.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
How do I take Melodetta 24 Fe?
-
Be sure to read these directions before you start taking your tablets or anytime you are not sure what to do.
- The tablets may be chewed and swallowed or swallowed whole. You should drink a full glass (8 ounces) of water immediately after chewing or swallowing.
- The right way to take the tablet is to take one tablet every day at the same time in the order directed on the package. Melodetta 24 Fe can be taken without regard to meals.
If you miss tablets you could get pregnant. This includes starting the pack late. The more tablets you miss, the more likely you are to get pregnant. See "What to Do if You Miss Tablets” below.
- Many women have spotting or light bleeding at unexpected times, or may feel sick to their stomach during the first 1 to 3 packs of tablets.
If you do have spotting or light bleeding or feel sick to your stomach, do not stop taking the tablets. The problem will usually go away. If it does not go away, check with your healthcare provider.
- Missing tablets can also cause spotting or light bleeding, even when you make up these missed tablets.
On the days you take two tablets, to make up for missed tablets, you could also feel a little sick to your stomach.
- If you have vomiting (within 3 to 4 hours after you take your tablet), you should follow the instructions for "What to Do if You Miss Tablets". If you have diarrhea or if you take certain medicines, including some antibiotics and some herbal products such as St. John's Wort, your tablets may not work as well.
Use a back-up method (such as condoms and spermicides) until you check with your healthcare provider.
- If you have trouble remembering to take Melodetta 24 Fe, talk to your healthcare provider about how to make tablet-taking easier or about using another method of birth control.
-
If you have any questions or are unsure about the information in this leaflet, call your healthcare provider.
Before You Start Taking Your Melodetta 24 Fe Tablets
1. Decide What Time of Day You Want to Take Your Tablet. It is important to take Melodetta 24 Fe tablets in the order directed on the package at the same time every day. Melodetta 24 Fe can be taken without regard to meals.
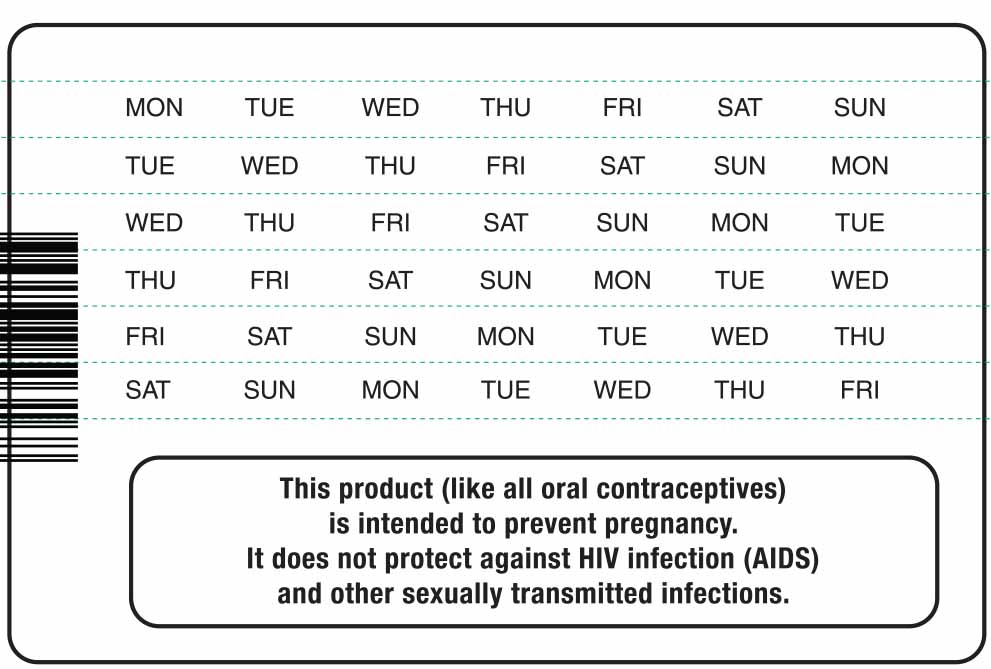
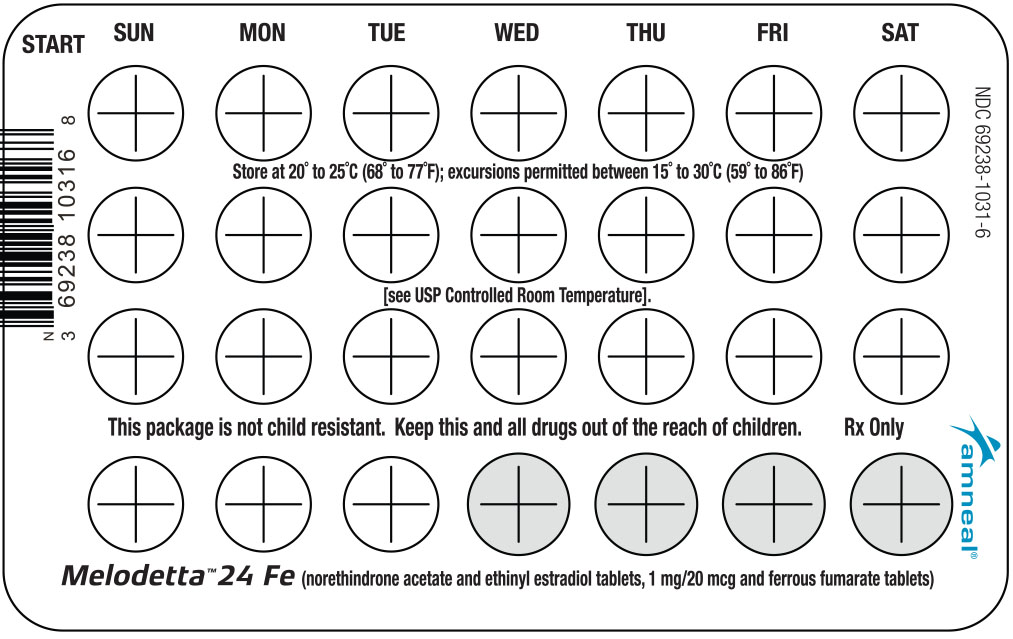
2. Look at Your Tablet Pack – It has 28 Tablets
The Melodetta 24 Fe pack has 24 "active" white tablets (with hormones) to be taken for 24 days, followed by 4 "reminder" brown tablets (without hormones) to be taken for the next four days.
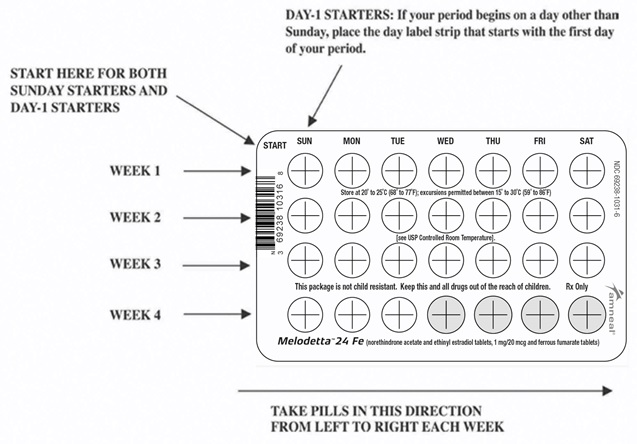
3. Also look for:
a) Where on the pack to start taking tablets,
b) In what order to take the tablets (follow the arrows shown in the picture above)
c) The week numbers as shown in the picture above.
4. Be sure you have ready at all times
a) another kind of birth control (such as a condoms and spermicide) to use as a back-up in case you miss tablets, and
b) an extra, full tablet pack.
When to Start the First Pack of Tablets
You have a choice for which day to start taking your first pack of tablets. Decide with your healthcare provider which is the best day for you. Pick a time of day which will be easy to remember.
Day 1 Start:
- Pick the day label strip that starts with the first day of your period (this is the day you start bleeding or spotting, even if it is almost midnight when the bleeding begins).
- Place this day label strip on the tablet dispenser over the area that has the days of the week (starting with Sunday) printed on the plastic.
- Take the first white tablet of the pack during the first 24 hours of your period.
- You will not need to use a back-up method of birth control, since you are starting the tablet at the beginning of your period. However, if you start Melodetta 24 Fe later than the first day of your period, you should use another method of birth control (such as a condom and spermicide) as a back-up method until you have taken 7 white tablets.
Sunday Start:
- Take the first white tablet of the pack on the Sunday after your period starts, even if you are still bleeding. If your period begins on Sunday, start the pack that same day.
- Use another method of birth control (such as a condom and spermicide) as a back-up method if you have sex anytime from the Sunday you start your first pack until the next Sunday (7 days). This also applies if you start Melodetta 24 Fe after having been pregnant, and you have not had a period since your pregnancy.
When You Switch From a Different Birth Control Tablet or Capsule
When switching from another birth control pill, finish all the tablets or capsules, then Melodetta 24 Fe should be started on the same day that a new pack of the previous birth control tablet or capsule would have been started.
When You Switch From Another Type of Birth Control Method
When switching from a transdermal patch or vaginal ring, finish the 21 days of use, wait 7 days, then Melodetta 24 Fe should be started when the next application would have been due. When switching from an injection, Melodetta 24 Fe should be started when the next injection would have been due. When switching from an intrauterine device or an implant, Melodetta 24 Fe should be started on the day of removal.
What to Do During the Month
- Take one tablet at the same time every day until the pack is empty.
Do not skip tablets even if you are spotting or bleeding between monthly periods or feel sick to your stomach (nausea).
- Do not skip tablets even if you do not have sex very often.
When you finish a pack of tablets, start the next pack on the day after your last brown tablet. Do not wait any days between packs.
What to Do if You Miss Tablets
Melodetta 24 Fe may not be as effective if you miss any white tablets, especially if you miss the first few or the last few white tablets in a pack.
If you miss 1 white tablet:
- Take the tablet as soon as you remember. Take the next tablet at your regular time. This means you may take two tablets in one day.
- You do not need to use a back-up birth control method if you have sex.
If you miss 2 white tablets in a row in week 1 OR week 2 of your pack:
- Take two tablets on the day you remember and two tablets the next day.
- Then take one tablet a day until you finish the pack.
-
You could become pregnant if you have sex in the 7 days after you restart your tablets. You must use another birth control method (such as a condom and spermicide) as a back-up for those 7 days.
If you miss 2 white tablets in a row in week 3 or week 4 of your pack:
-
If you are a Day 1 Starter:
Throw out the rest of the tablet pack and start a new pack that same day.
If you are a Sunday Starter:
Keep taking one tablet every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack of tablets that same day.
-
You could become pregnant if you have sex in the 7 days after you restart your tablets. You must use another birth control method (such as a condom and spermicide) as a back-up for those 7 days.
- You may not have your period this month but this is expected. However, if you miss your period two months in a row, call your healthcare provider because you might be pregnant.
If you miss 3 or more white tablets in a row during any week:
-
If you are a Day 1 Starter:
Throw out the rest of the tablet pack and start a new pack that same day.
If you are a Sunday Starter:
Keep taking 1 tablet every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack of tablets that same day.
-
You could become pregnant if you have sex on the days when you missed tablets or during the first 7 days after you restart your tablets. You must use another birth control method (such as a condom and spermicide) as a back-up the next time you have sex and for the first 7 days after you restart your tablets.
- You may not have your period this month but this is expected. However, if you miss your period two months in a row, call your healthcare provider because you might be pregnant.
If you miss any of the 4 brown tablets in Week 4:
- Throw away the tablets you missed.
- Keep taking one tablet each day until the pack is empty.
- You do not need a back-up method.
- Start the next pack of Melodetta 24 Fe as scheduled.
Finally, if you are still not sure what to do about the tablets you have missed:
- Use a back-up method (such as a condom and spermicide) anytime you have sex.
- Contact your healthcare provider and continue taking one active white tablet each day until otherwise directed.
Who should not take Melodetta 24 Fe?
Your healthcare provider will not give you Melodetta 24 Fe if you have:
- Ever had blood clots in your arms, legs (deep vein thrombosis), lungs (pulmonary embolism), or eyes (retinal thrombosis)
- Ever had a stroke
- Ever had a heart attack
- Certain heart valve problems or heart rhythm abnormalities that can cause blood clots to form in the heart
- An inherited problem with your blood that makes it clot more than normal
- High blood pressure that medicine cannot control
- Diabetes with kidney, eye, nerve, or blood vessel damage
- Ever had certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision, or have any migraine headache if you are over age 35.
- Ever had breast cancer, which may be sensitive to female hormones
- Liver disease, including liver tumors
- Take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood
Also, do not take birth control pills if you:
- Smoke and are over 35 years old
- Are or suspect you are pregnant
- Have any unexplained bleeding from the vagina
Birth control pills may not be a good choice for you if you have ever had jaundice (yellowing of the skin or eyes) caused by pregnancy, also called cholestasis of pregnancy.
Tell your healthcare provider if you have ever had any of the above conditions (your healthcare provider may recommend another method of birth control).
What else should I know about taking Melodetta 24 Fe?
Birth control pills do not protect you against any sexually transmitted infection, including HIV, the virus that causes AIDS.
Do not skip any tablets, even if you do not have sex often.
If you miss a period, you could be pregnant. However, some women miss periods or have light periods on birth control pills, even when they are not pregnant. Contact your healthcare provider for advice if you:
- Think you are pregnant
- Miss one period and have not taken your birth control pills every day
- Miss two periods in a row
Birth control pills should not be taken during pregnancy. However, birth control pills taken by accident during pregnancy are not known to cause birth defects.
You should stop Melodetta 24 Fe at least four weeks before you have surgery and not restart it until at least two weeks after the surgery, due to an increased risk of blood clots.
If you are breastfeeding, consider another birth control method until you are ready to stop breastfeeding. Birth control pills that contain estrogen, like Melodetta 24 Fe, may decrease the amount of milk you make. A small amount of the pill's hormones passes into breast milk.
Tell your healthcare provider about all medicines and herbal products that you take. Some medicines and herbal products may make birth control pills less effective, including:
- barbiturates
- bosentan
- carbamazepine
- felbamate
- griseofulvin
- oxcarbazepine
- phenytoin
- rifampin
- St. John’s wort
- topiramate
Use a back-up or alternative birth control method when you take medicines that may make birth control pills less effective.
Birth control pills may interact with lamotrigine, an anticonvulsant used for epilepsy. This may increase the risk of seizures, so your healthcare provider may need to adjust the dose of lamotrigine.
If you have vomiting or diarrhea, your birth control pills may not work as well. Use another birth control method, like a condom and spermicide, until you check with your healthcare provider.
Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone.
If you are scheduled for any laboratory tests, tell your healthcare provider that you are taking birth control pills. Certain blood tests may be affected by birth control pills.
What are the most serious risks of taking Melodetta 24 Fe?
Like pregnancy, birth control pills increase the risk of serious blood clots, especially in women who have other risk factors, such as smoking, obesity, or age greater than 35. This increased risk is highest when you first start taking birth control pills and when you restart the same or different birth control pills after not using them for a month or more.
It is possible to die from a problem caused by a blood clot, such as a heart attack or a stroke.
Some examples of serious blood clots are blood clots in the:
- Legs (deep vein thrombosis)
- Lungs (pulmonary embolus)
- Eyes (loss of eyesight)
- Heart (heart attack)
- Brain (stroke)
Women who take birth control pills may get:
- High blood pressure
- Gallbladder problems
- Rare cancerous or noncancerous liver tumors
All of these events are uncommon in healthy women.
Call your healthcare provider right away if you have:
- Persistent leg pain
- Sudden shortness of breath
- Sudden blindness, partial or complete
- Severe pain or pressure in your chest
- Sudden, severe headache unlike your usual headaches
- Weakness or numbness in an arm or leg, or trouble speaking
- Yellowing of the skin or eyeballs
What are the common side effects of birth control pills?
The most common side effects of birth control pills are:
- Spotting or bleeding between menstrual periods
- Nausea
- Breast tenderness
- Headache
These side effects are usually mild and usually disappear with time.
Less common side effects are:
- Acne
- Less sexual desire
- Bloating or fluid retention
- Blotchy darkening of the skin, especially on the face
- High blood sugar, especially in women who already have diabetes
- High fat (cholesterol, triglyceride) levels in the blood
- Depression, especially if you have had depression in the past. Call your healthcare provider immediately if you have any thoughts of harming yourself
- Problems tolerating contact lenses
- Weight gain
This is not a complete list of possible side effects. Talk to your healthcare provider if you develop any side effects that concern you. You may report side effects to the FDA at 1-800-FDA-1088.
No serious problems have been reported from a birth control pill overdose, even when accidentally taken by children.
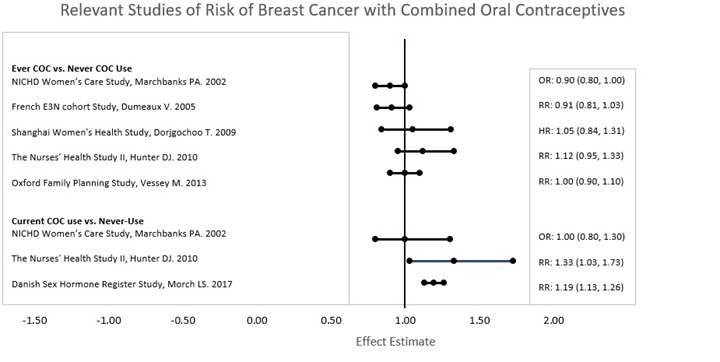
Do birth control pills cause cancer?
It is not known if hormonal birth control pills cause breast cancer. Some studies, but not all, suggest that there could be a slight increase in the risk of breast cancer among current users with longer duration of use.
If you have breast cancer now, or have had it in the past, do not use hormonal birth control because some breast cancers are sensitive to hormones.
Women who use birth control pills may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners.
What should I know about my period when taking Melodetta 24 Fe?
Irregular vaginal bleeding or spotting may occur while you are taking Melodetta 24 Fe. Irregular bleeding may vary from slight staining between menstrual periods to breakthrough bleeding, which is a flow much like a regular period. Irregular bleeding occurs most often during the first few months of oral contraceptive use, but may also occur after you have been taking the pill for some time. Such bleeding may be temporary and usually does not indicate any serious problems. It is important to continue taking your tablets on schedule. If the bleeding occurs in more than one cycle, is unusually heavy, or lasts for more than a few days, call your healthcare provider.
Some women may not have a menstrual period but this should not be cause for alarm as long as you have taken the tablets according to direction.
What if I miss my scheduled period when taking Melodetta 24 Fe?
It is not uncommon to miss your period. However, if you go two or more months in a row without a period, or you miss your period after a month where you did not take all your tablets correctly, call your healthcare provider because you may be pregnant. Also notify your healthcare provider if you have symptoms of pregnancy such as morning sickness or unusual breast tenderness. Stop taking Melodetta 24 Fe if you are pregnant.
What if I want to become pregnant?
You may stop taking the tablets whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop taking the tablets.
General Advice about Melodetta 24 Fe
Your healthcare provider prescribed Melodetta 24 Fe for you. Please do not share Melodetta 24 Fe with anyone else. Keep Melodetta 24 Fe out of the reach of children.
If you have concerns or questions, ask your healthcare provider. You may also ask your pharmacist for a more detailed label written for healthcare professionals.
For all medical inquiries contact Amneal Pharmaceuticals at 1-877-835-5472.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 06-2023-05
Close
 ...
...