Label: LUZU- luliconazole cream
- NDC Code(s): 99207-850-02, 99207-850-60
- Packager: Bausch Health US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LUZU safely and effectively. See full prescribing information for LUZU. LUZU® (luliconazole) cream, for topical use - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGELUZU (luliconazole) Cream, 1% is indicated for the topical treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organisms Trichophyton rubrum and Epidermophyton ...
-
2 DOSAGE AND ADMINISTRATIONFor topical use only. LUZU Cream, 1% is not for ophthalmic, oral, or intravaginal use. • When treating interdigital tinea pedis, a thin layer of LUZU Cream, 1% should be applied to the affected ...
-
3 DOSAGE FORMS AND STRENGTHSCream, 1%. Each gram of LUZU Cream, 1% contains 10 mg of luliconazole in a white cream base.
-
4 CONTRAINDICATIONSNone.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSAn in vivo study in adult subjects with moderate to severe interdigital tinea pedis and tinea cruris showed that LUZU Cream, 1% is mostly a weak inhibitor of CYP2C19. In a separate trial in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with LUZU Cream, 1% use in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. In animal ...
-
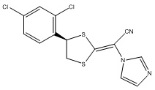
11 DESCRIPTIONLUZU (luliconazole) Cream, 1% contains 1% luliconazole, an azole antifungal agent, in a white cream for topical application. Luliconazole is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - LUZU Cream, 1% is an azole antifungal [seeMicrobiology (12.4)]. 12.2 Pharmacodynamics - At therapeutic doses, LUZU Cream, 1% is not expected to prolong QTc to any ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate the carcinogenic potential of LUZU Cream, 1% have not been conducted. Luliconazole revealed no evidence ...
-
14 CLINICAL STUDIES14.1 Interdigital Tinea Pedis - The safety and efficacy of LUZU (luliconazole) Cream, 1% was evaluated in two randomized, double-blind, vehicle-controlled, multi-center clinical trials in 423 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLUZU (luliconazole) Cream, 1% is a white cream supplied in tubes as follows: 60 g NDC 99207-850-60 - Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). • Inform patients that LUZU Cream, 1% is for topical use only. LUZU Cream, 1% is not intended for intravaginal ...
-
PATIENT INFORMATION LUZU® (loo-zoo) (luliconazole) Cream, 1% Important information: LUZU Cream is for use on skin only. Do not get LUZU Cream near or in your eyes, mouth or vagina. What is LUZU ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 60 g Carton - NDC 99207-850-60 - Rx only - LUZU® (luliconazole) Cream, 1% For Topical Use Only - Not for ophthalmic, oral, or intravaginal use - Keep Out of Reach of ...
-
INGREDIENTS AND APPEARANCEProduct Information