Label: LUXTURNA- voretigene neparvovec-rzyl kit
- NDC Code(s): 71394-065-01, 71394-415-01, 71394-716-01
- Packager: Spark Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated April 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LUXTURNA safely and effectively. See full prescribing information for LUXTURNA. LUXTURNA (voretigene neparvovec-rzyl) intraocular ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGELUXTURNA (voretigene neparvovec-rzyl) is an adeno-associated virus vector-based gene therapy indicated for the treatment of patients with confirmed biallelic RPE65 mutation-associated retinal ...
-
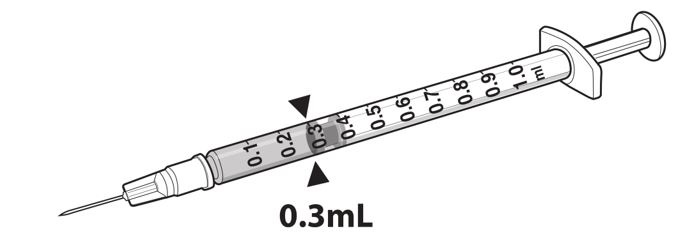
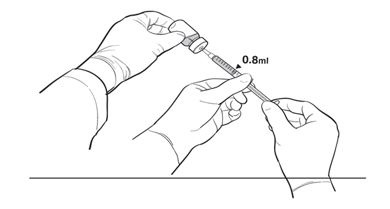
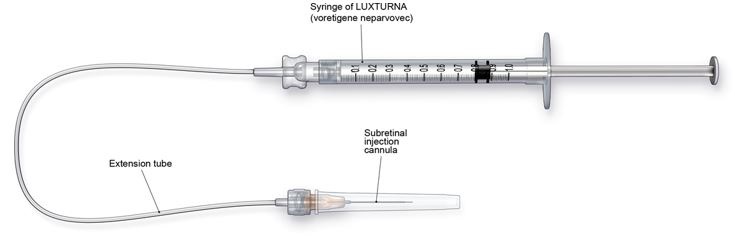
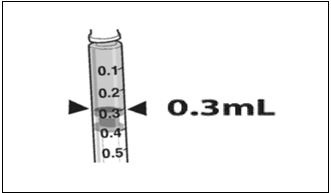
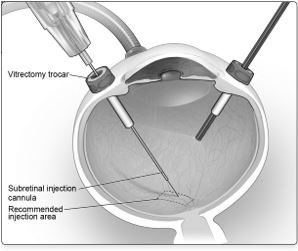
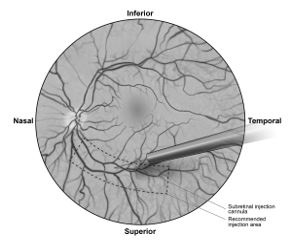
2 DOSAGE AND ADMINISTRATIONFor subretinal injection only. 2.1 Dose - The recommended dose of LUXTURNA for each eye is 1.5 x 1011 vector genomes (vg), administered by subretinal injection in a total volume of 0.3 ...
-
3 DOSAGE FORMS AND STRENGTHSLUXTURNA is a suspension for subretinal injection, supplied in a 0.5-mL extractable volume in a 2-mL single-dose vial; the supplied concentration (5 x 1012 vg/mL) requires a 1:10 dilution prior to ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Endophthalmitis - Endophthalmitis may occur following any intraocular surgical procedure or injection. Use proper aseptic injection technique when administering LUXTURNA. Following the ...
-
6 ADVERSE REACTIONSThe most common adverse reactions (incidence ≥ 5%) were conjunctival hyperemia, cataract, increased intraocular pressure, retinal tear, dellen (thinning of the corneal stroma), macular hole ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Adequate and well-controlled studies with LUXTURNA have not been conducted in pregnant women. Animal reproductive studies have not been conducted with LUXTURNA ...
-
11 DESCRIPTIONLUXTURNA (voretigene neparvovec-rzyl) is a suspension of an adeno-associated virus vector-based gene therapy for subretinal injection. LUXTURNA is a live, non-replicating adeno-associated virus ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - LUXTURNA is designed to deliver a normal copy of the gene encoding the human retinoid isomerohydrolase RPE65 (RPE65) to cells of the retina in persons with reduced or ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No animal studies have been conducted to evaluate the effects of LUXTURNA on carcinogenesis, mutagenesis, and impairment of ...
-
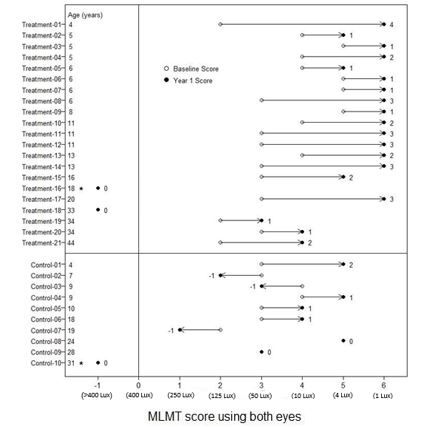
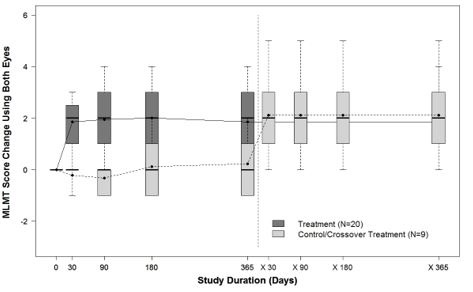
14 CLINICAL STUDIESThe efficacy of LUXTURNA in pediatric and adult patients with biallelic RPE65 mutation-associated retinal dystrophy was evaluated in an open-label, two-center, randomized trial (Study 2). Of the ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach carton of LUXTURNA (NDC 71394 – 415-01) contains one single-dose vial of the LUXTURNA (NDC 71394 – 065-01, 0.5 mL extractable volume) and two vials of Diluent (NDC 71394 – 716-01, 1.7 mL ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients and/or their caregivers of the following risks: Endophthalmitis and other eye infections - Serious infection can occur inside of the eye and may lead to blindness. In such ...
-
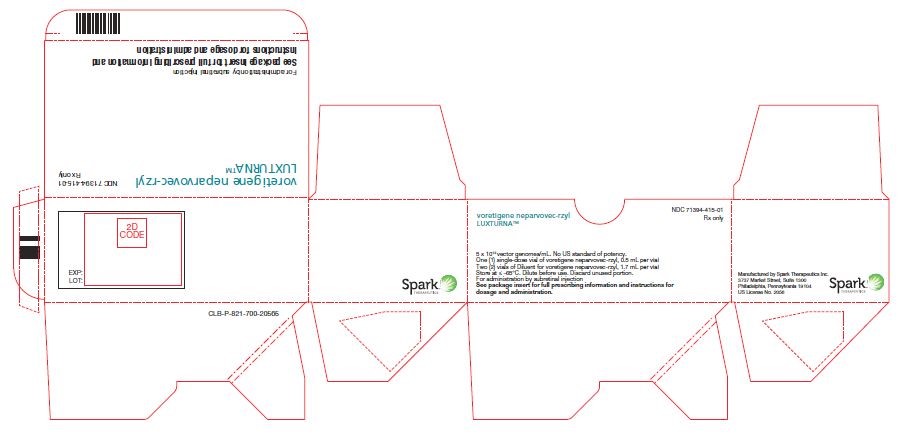
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Luxturna Carton Label - NDC 71394-415-01 - Rx only - voretigene neparvovec-rzyl - LUXTURNA™ 5 x 1012 vector genomes/mL. No US standard of potency. One (1) single-dose vial of ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Diluent for Voretigene Neparvovec Label - NDC 71394-716-01 - Diluent for voretigene neparvovec-rzyl - pH 7.3, containing 0.001% poloxamer 188 - For Rx only - 1.7 mL per vial ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Luxturna Label - NDC 71394-065-01 - voretigene neparvovec-rzyl - For subretinal injection - 5 x 1012 vector genomes/mL - One (1) single-dose vial, 0.5 mL per vial - For Rx only ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Luxturna Pouch Label - NDC 71394-415-01 - Rx only - voretigene neparvovec-rzyl - LUXTURNA™ 5 x 1012 vector genomes/mL. No US standard of potency. One (1) single-dose vial of ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Luxturna Pouch Label - NDC 71394-415-01 - Rx only - voretigene neparvovec-rzyl - LUXTURNA™ 5 x 1012 vector genomes/mL. No US standard of potency. One (1) single-dose vial of ...
-
INGREDIENTS AND APPEARANCEProduct Information