Label: ZEPZELCA- lurbinectedin injection, powder, lyophilized, for solution
- NDC Code(s): 68727-712-01
- Packager: Jazz Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZEPZELCA safely and effectively. See full prescribing information for ZEPZELCA. ZEPZELCA® (lurbinectedin) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE ZEPZELCA is indicated for the treatment of adult patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy. This indication is ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage - The recommended dosage of ZEPZELCA is 3.2 mg/m2 by intravenous infusion over 60 minutes every 21 days until disease progression or unacceptable toxicity. Initiate ...
-
3 DOSAGE FORMS AND STRENGTHS For injection: 4 mg of lurbinectedin as a sterile, preservative-free, white to off-white lyophilized powder in a single-dose vial for reconstitution prior to intravenous infusion.
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Myelosuppression - ZEPZELCA can cause myelosuppression. In clinical studies of 554 patients with advanced solid tumors receiving ZEPZELCA [see Adverse Reactions (6.1)], Grade 3 or 4 ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Myelosuppression [see Warnings and Precautions (5.1)] • Hepatotoxicity [see Warnings and ...
-
7 DRUG INTERACTIONS 7.1 Effect of Other Drugs on ZEPZELCA - Strong and Moderate CYP3A Inhibitors - Coadministration of ZEPZELCA with a strong or a moderate CYP3A inhibitor increases lurbinectedin systemic exposure ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on animal data and its mechanism of action [see Clinical Pharmacology (12.1)], ZEPZELCA can cause fetal harm when administered to a pregnant woman. There are ...
-
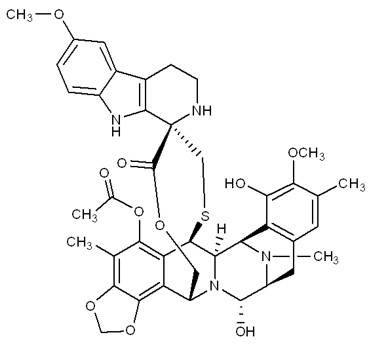
11 DESCRIPTION ZEPZELCA is an alkylating drug. The chemical name of ZEPZELCA (lurbinectedin) is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Lurbinectedin is an alkylating drug that binds guanine residues in the minor groove of DNA, forming adducts and resulting in a bending of the DNA helix towards the ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity testing of lurbinectedin has not been performed. Lurbinectedin is genotoxic to mammalian cells in the presence and ...
-
14 CLINICAL STUDIES PM1183-B-005-14 (Study B-005; NCT02454972) is a multicenter, open-label, multi-cohort trial evaluating ZEPZELCA as a single agent in patients with advanced or metastatic solid tumors. A cohort of ...
-
15 REFERENCES 1. "OSHA Hazardous Drugs." OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied - ZEPZELCA (lurbinectedin) for injection is supplied as a sterile, preservative-free, white to off-white lyophilized powder in a single-dose clear glass vial. Each carton (NDC ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Myelosuppression - Advise patients to immediately contact their healthcare provider for fever, other signs of ...
-
Patient Package Insert PATIENT INFORMATION - ZEPZELCA® (zep zel' kah) (lurbinectedin) for injection - What is ZEPZELCA? • ZEPZELCA is used to treat adults with a kind of lung cancer called small cell ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Carton: NDC 68727-712-01 - ZEPZELCA - (lurbinectedin) for injection - 4 mg per vial - FOR INTRAVENOUS INFUSION ONLY - Reconstitute before further dilution. Each single-dose vial contains 4 mg of ...
-
Package/Label Display Panel Vial: NDC 68727-712-01 - ZEPZELCA - (lurbinectedin) for injection - 4 mg per vial - FOR INTRAVENOUS INFUSION ONLY - Single-Dose Vial. Discard unused portion.
-
INGREDIENTS AND APPEARANCEProduct Information