Label: LUMOXITI- moxetumomab pasudotox injection, powder, lyophilized, for solution
IV STABILIZER- polysorbate 80 solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 73380-4700-1, 73380-4715-9 - Packager: Innate Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated September 3, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LUMOXITI safely and effectively. See full prescribing information for LUMOXITI. LUMOXITI® (moxetumomab pasudotox-tdfk) for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CAPILLARY LEAK SYNDROME and HEMOLYTIC UREMIC SYNDROME
- Capillary Leak Syndrome (CLS), including life-threatening cases, occurred in patients receiving LUMOXITI. Monitor weight and blood pressure; check labs, including albumin, if CLS is suspected. Delay dosing or discontinue LUMOXITI as recommended [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
- Hemolytic Uremic Syndrome (HUS), including life-threatening cases, occurred in patients receiving LUMOXITI. Monitor hemoglobin, platelet count, serum creatinine, and ensure adequate hydration. Discontinue LUMOXITI in patients with HUS [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGELUMOXITI is indicated for the treatment of adult patients with relapsed or refractory hairy cell leukemia (HCL) who received at least two prior systemic therapies, including treatment with a ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dose of LUMOXITI is 0.04 mg/kg administered as a 30-minute intravenous infusion on Days 1, 3, and 5 of each 28-day cycle. Continue LUMOXITI treatment for ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 1 mg as a white to off-white lyophilized cake or powder in a single-dose vial for reconstitution and further dilution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Capillary Leak Syndrome (CLS) Capillary leak syndrome (CLS), including life-threatening cases, has been reported among patients treated with LUMOXITI and is characterized by ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling. Capillary Leak Syndrome [see Warnings and Precautions (5.1)] Hemolytic Uremic Syndrome [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and findings in non-pregnant female animals, LUMOXITI is expected to cause maternal and embryo-fetal toxicity when administered ...
-
11 DESCRIPTIONMoxetumomab pasudotox-tdfk is a CD22-directed cytotoxin. Moxetumomab pasudotox-tdfk is composed of a recombinant, murine immunoglobulin variable domain genetically fused to a truncated form of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Moxetumomab pasudotox-tdfk is a CD22-directed cytotoxin. Moxetumomab pasudotox-tdfk binds CD22 on the cell surface of B-cells and is internalized. Moxetumomab ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted to assess the carcinogenic or genotoxic potential of moxetumomab pasudotox-tdfk. Animal fertility ...
-
14 CLINICAL STUDIESThe efficacy of LUMOXITI was based upon Study 1053 titled "A Pivotal Multicenter Trial of Moxetumomab Pasudotox in Relapsed/Refractory Hairy Cell Leukemia" (NCT01829711). Study 1053 was conducted ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - LUMOXITI (moxetumomab pasudotox-tdfk) for injection is supplied as a sterile, preservative-free, white to off-white lyophilized cake or powder in a 1 mg single-dose vial. Each ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Capillary Leak Syndrome - Advise patients on the risk of developing capillary leak syndrome. Advise patients to ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Innate Pharma Inc., Rockville, MD 20850 - Manufactured by: Innate Pharma Inc., Rockville, MD 20850 - U.S. License No. 2206 - LUMOXITI is a registered trademark of Innate Pharma. © Innate ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Issued: August 2020 - MEDICATION GUIDE - LUMOXITI® (loo-MOCKS-eh-tee) (moxetumomab pasudotox-tdfk) for ...
-
INSTRUCTIONS FOR USELUMOXITI® (moxetumomab pasudotox-tdfk) for injection - Healthcare Provider Instructions for Use - Important Information - Read the following instructions before reconstitution, dilution, and ...
-
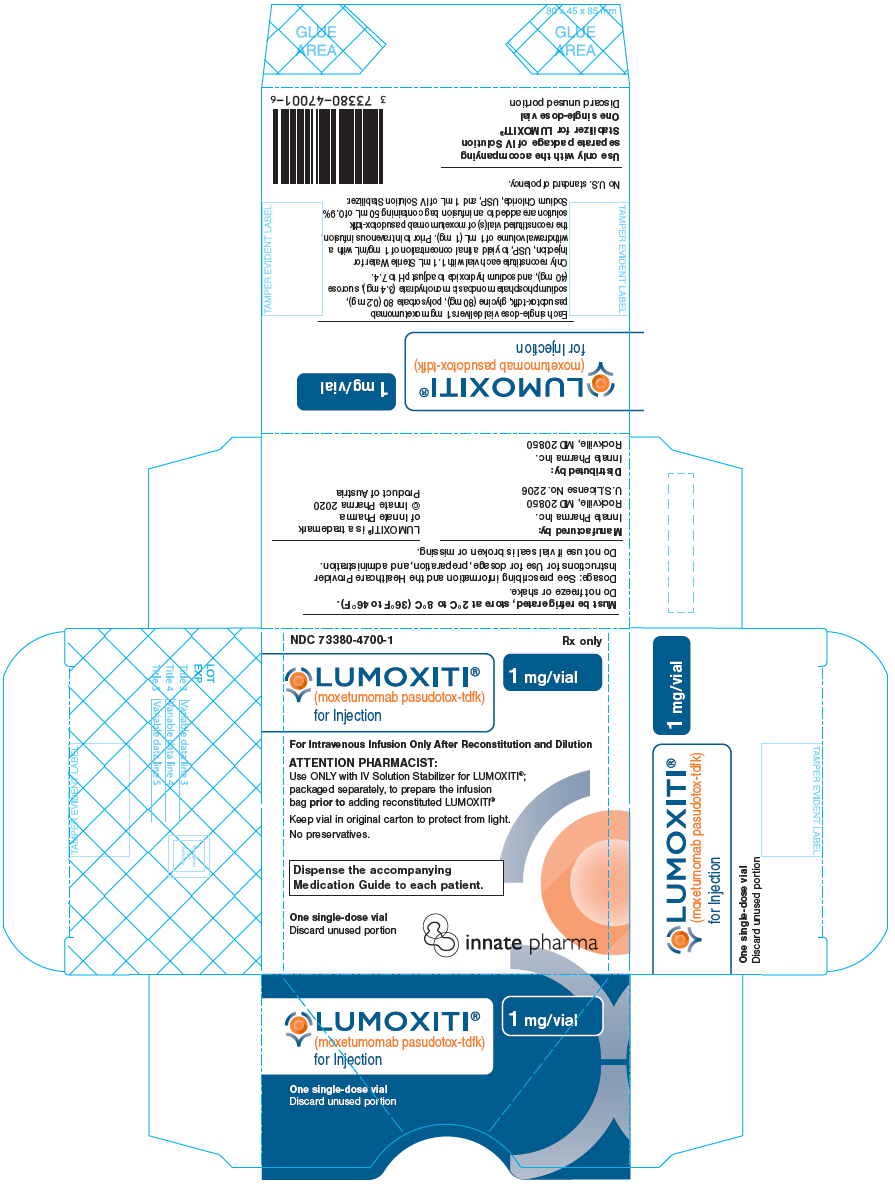
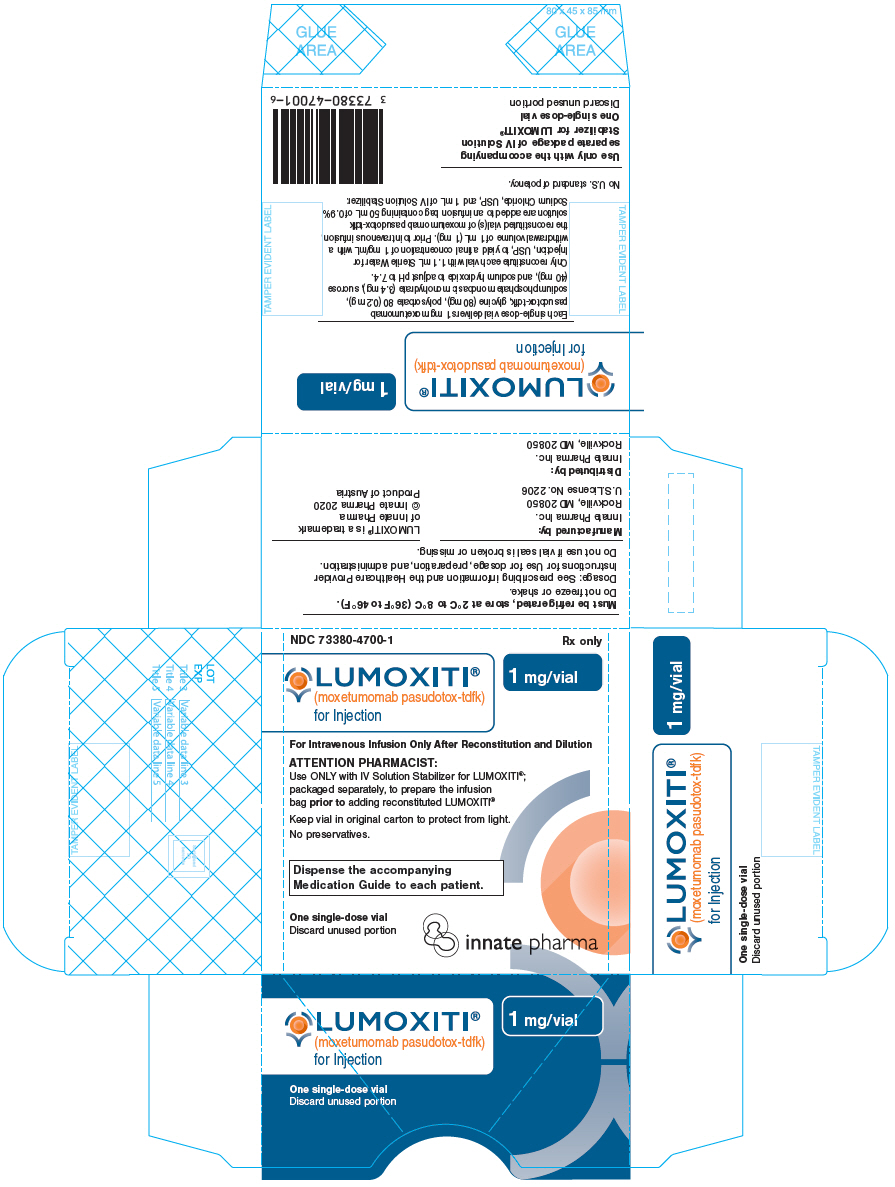
PRINCIPAL DISPLAY PANEL - 1 mg Vial CartonNDC 73380-4700-1 - Rx only - LUMOXITI® (moxetumomab pasudotox-tdfk) for Injection - 1 mg/vial - For Intravenous Infusion Only After Reconstitution and Dilution - ATTENTION PHARMACIST: Use ONLY with IV ...
-
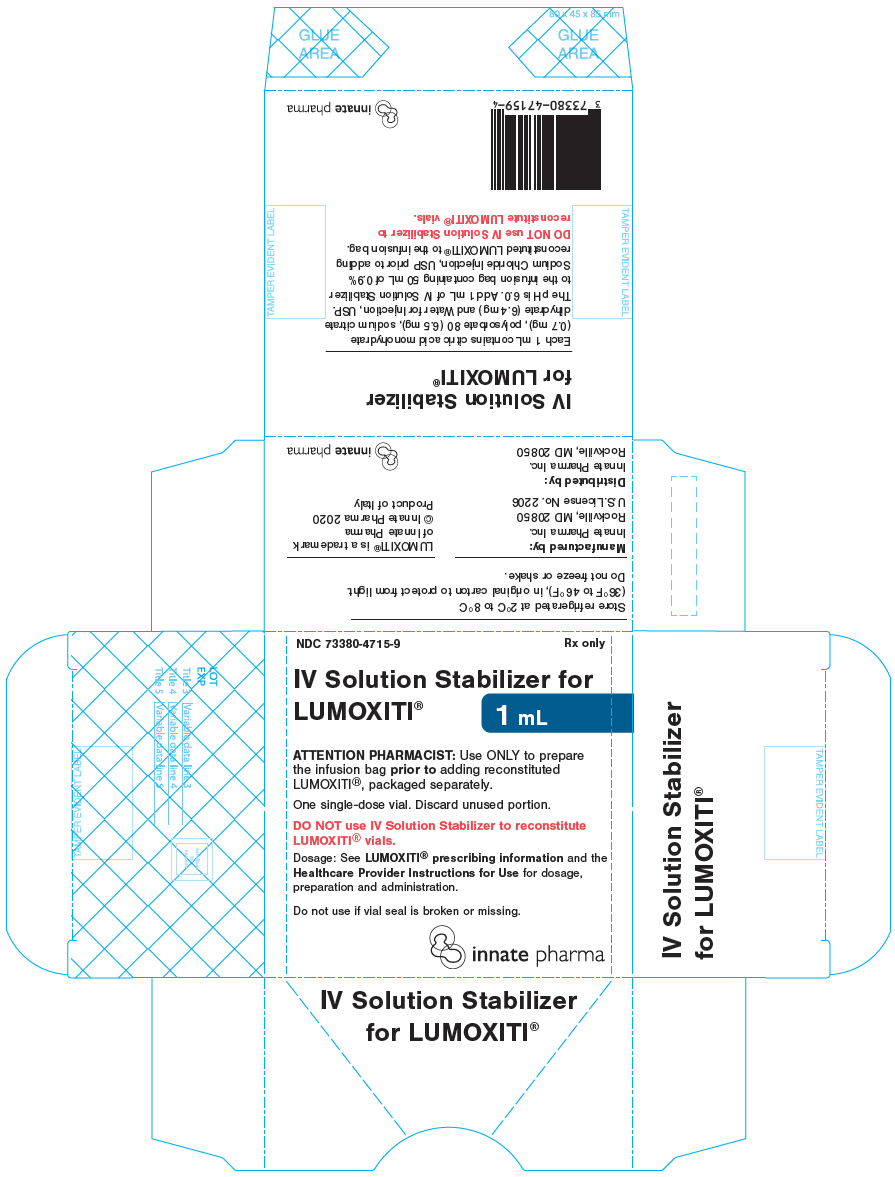
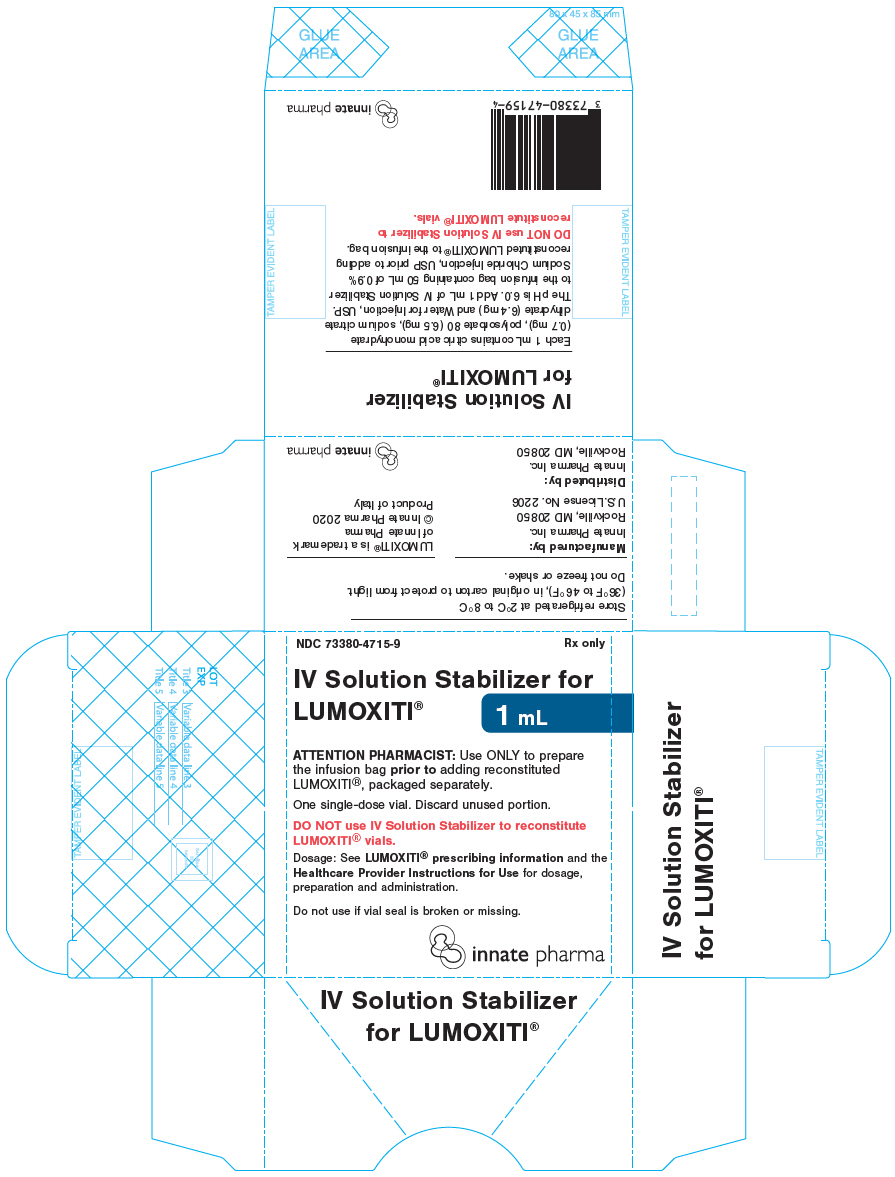
PRINCIPAL DISPLAY PANEL - 1 mL Vial CartonNDC 73380-4715-9 - Rx only - IV Solution Stabilizer for - LUMOXITI® 1 mL - ATTENTION PHARMACIST: Use ONLY to prepare - the infusion bag prior to adding reconstituted - LUMOXITI®, packaged separately. One ...
-
INGREDIENTS AND APPEARANCEProduct Information